61
- Principles of Clinical Pharmacology NIH
- Instant Clinical Pharmacology Evan Begg 2008
- pharmacology 2000
- How Drugs Work Hugh McGavock 2010
- Medicinesforchildren.org.uk
- MHRA Drug safety updates
- iTU pharmacology part 1 alexa george
- iTU pharmacology part 2 alexa george
- iTU receptors alexa george
Prescribing information sources
- eBNF
- eMedicines Compendium
- Medicines and Healthcare Products Regulatory Agency
- National Prescribing Centre
- Prescription Pricing Authority
- National Electronic Library for Medicines
- DH Health Information for Overseas Travellers (Yellow Book)
- DH Immunisation Against Infectious Disease. HMSO, 1996 (Green Book)
- MHRA Guidelines for prescibing in children
- PABG OTC directory online
- Drug and Therapeutics Bulletin
- NPC MeReC bulletins
- PPA Drug Tariff – Useful for troublesome prescriptions for stoma bags, dressings, catheters, etc
- Poisons Information Services
- MIMS Poisons Information
- NHS BSAObtaining a BNF
- www.medicines.org.uk
- www.emims.net
- www.palliativedrugs.com
- Palliative Medicine Handbook
- Practice or local PCO prescribing protocol or formulary
- Local pharmacists
- Hospital pharmacists and drug information services
- PCA prescribing advisor
- Regional and district drug information services
- Drug companies
Clinical Pharmacology
| Pharmacokinetics and Pharmacodynamics |
|---|
| PK first then PD |
| Pharmacokinetics |
drug metabolism – how the tissues process the drug
|
| Pharmacodynamics |
| physiological/pharmocological effects of the drug on the substrate tissues (including desired effects and side effects) |
| PKPD of Drug interactions | |
|---|---|
| PK |
|
| PD | drugs having similar physiological/pharmacological actions at the site of action
|
| From Davidsons Foundations of Clinical Practice | |
| Pharmacogenomics |
|---|
Drug Dose Calculations
| Body surface area |
|---|
| = square root (ht cm x wt kg / 3600) |
| Body Surface Area Calculator (Dubois) BNF plus |
| Drip Rates | ||
|---|---|---|
|
||
| 1000ml | ml/hr | drops/min |
| q2h | 500 | 167 |
| q4h | 250 | 83 |
| q6h | 167 | 56 |
| q8h | 125 | 42 |
| q10h | 100 | 33 |
| q12h | 83 | 28 |
| q16h | 63 | 21 |
| q24h | 42 | 14 |
| Prescribing cautions in specific populations |
|---|
|
Always check if a woman is, or might be, pregnant or breast feeding
Prescribing in pregnancy
- tga.gov.au medicines in pregnancy
- Medicines in pregnancy bpac.org.nz
- scribd.com Prescribing in Pregnancy Rubin and Ramsay
- BNF
Drugs and breast feeding
Breastfeeding mothers should seek advice on the suitability of OTC products before taking.
Assess the benefit/risk ratio for both mother and infant
Avoid use of drugs known to cause serious toxicity in adults or children
Drugs licensed for use in infants do not generally pose a hazard
Neonates (and particularly premature infants) are at greater risk from exposure to drugs via breast milk, because of immature excretory functions and the consequent risk of drug accumulation
Choose a regimen and route of administration which presents the minimum amount of drug to the infant
It is best to avoid long-acting preparations, especially those of drugs likely to cause serious side effects (e.g. antipsychotic agents), as it is difficult to time feeds to avoid significant amounts of drug in breast milk
Multiple drug regimens may pose an increased risk especially when adverse effects such as drowsiness are additive
Infants exposed to drugs via breast milk should be monitored for unusual signs or symptoms Avoid new drugs if an alternative that has been more widely used is available.
Avoid
- Phenothiazine derivatives
- Salicylates
- Tetracyclines
- Corticosteroids in higher dose (> 10 mg/day)
- Thyroxine
- Warfarin
- Indomethacin
- Cotrimoxazole
- Ergot/ergotamine preparations
- Oral hypoglycaemics
- Nalidixic acid
- oestrogens ie COCP
Common drugs to avoid include:
- Aminophylline
- betablockers
- Benzodiazepines in higher doses
- Carbimazole
- Naproxen
- Nitrazepam
- Paracetamol
- Penicillins
- Trimethoprim
- Erythromycin
- Insulin
Drugs reasonably safe in breast feeding
- Antacids
- Antihistamines
- Chlormethiazole
- Cimetidine
- Codeine
- Digoxin
- Heparin
- Methyldopa
Prescribing for children
- cBNF @ bnf.org
- Bamboozled by cBNF A Cox ncbi.nlm.nih
- Prescribing in Children PUK
- Medicines for children.org.uk
- Medicines for children MHRA
- Prescribing for children MeReC 2000 pdf
- MPS Common Problems in Hospital Setting
- NeLM Making medicines safer for children – use of unlicensed medicines in paeds patients
- Safe prescribing for children? Arch Dis Child 2008
Prescribe according to local paediatric formulary.
Unlicensed and Off-label Medicines in children
You may need to use unlicensed medicines or licensed medicines for unlicensed purposes when prescribing in children.
In such cases the parent/carer and child, where appropriate, should be fully informed and agreeable to the treatment.
Most doses are given for age ranges or weight.
In primary care you will rarely need to prescribe according to body surface area but there is a conversion table at the back of the BNF.
Body Surface Area Calculator (Dubois) BNF plus
Get a colleague to double-check any dose calculations you make.
Advise parents of the indications for the medicine and of potential side effects, and remind them of safe storage of all medicines.
Check for family history of severe penicillin allergy before prescribing for the first time.
The standard 5 ml medicine spoon is supplied where the dose is a multiple of 5 ml, otherwise the pharmacist will supply a medicine syringe. Advise parents to place the syringe between the cheek and gum margin, not directly on the tongue, with the child held at 45 degrees. Drugs shouldn’t be added to bottles or feeds.
Prescribing in the elderly
- STOPP START
- STOPP START Cumbria
- Inappropriate Prescribing BSG Mahony
- STOPP Criteria
- NELM: Health Foundation report: Making care safer Jul 2011
- Download Report pdf
- Pulse CPD Module 2010 Therapeutics in the Elderly
- Duke Clinical Research Beers Criteria Medication List
- Beers Criteria Pharmacy Times 2006
- STOPP Using Beers’ List?
- Care Homes Internal Link
Drug Problems in the Elderly
drug interactions
confusion
Hyperkalaemia
hyponatraemia
NSAIDs
Physiological changes
decreased salivation and swallowing, protein binding, drug metabolism/elimination
altered drug-tissue distribution and drug-tissue responses
unavoidable polypharmacy (if multiple pathology is present)
poor medication compliance (often).
The aging process healthinaging.org
Biological age
altered liver and kidney function,
nutrition,
tissue responses
body composition.
Metabolic Changes (McGavock)
Liver changes
Decreased blood flow leads to decreased presystemic drug metabolism
Decreased liver size, microsomal (P450) oxidation and antipyrine
clearances lead to decreased hepatic drug metabolism
Kidney changes
The number of nephrons decreases by 6% per decade; although serum creatinine may be normal, older people do have reduced renal function; at 70
years of age, renal function is, at best, 50% of its original maximum. Check the eGFR.
Decreased glomerular filtration rate and tubular secretion lead to an increased possibility of accumulation of all drugs and metabolites eliminated via the kidneys.
NSAIDs can accelerate the decline in renal function, particularly in the presence of cardiac failure by inhibiting renal prostaglandin
synthesis, causing tubular ischaemia and retention of sodium and water,
which may in turn precipitate or worsen left ventricular failure
If standard adult doses of many drugs are given to this age group, excessive plasma concentrations will gradually accumulate. The problem is exacerbated by reduced kidney function and a resulting reduction in the ability to excrete drugs and their metabolites. The plasma half-lives of digoxin, lithium and gentamicin are doubled, while that of diazepam maybe quadrupled.
Nutrition changes
Vitamins decrease
Proteins decrease
Tissue response changes
Reduction in the number of brain cells increases effects of psychoactive drugs
Reduction in baroreceptor activity increases postural hypotensive effect of drugs
Exaggerated response to anticoagulants; increased risk of gastrointestinal bleeding with NSAIDs
Changes in body composition
Reduced body weight, body water and plasma albumin – increasing the plasma drug concentration and the effect of many medicines
Decreased body weight
Decreased body water
Increased body fat percentage
Decreased plasma albumin
Result
Increased effect of standard dose
Increased plasma concentration of water-soluble drugs
Decreased plasma concentration of fat-soluble drugs
Reduced protein binding
Questions to consider when reviewing the current long-term drug treatment of elderly patients (Mcgavock)
- Is the medication strictly necessary?
- Is it being taken?
- Are there any side-effects?
- Is it having any therapeutic effect?
- Are any of the drugs incompatible?
- Are there signs of drug-drug interaction?
Once or twice-daily regimens may improve compliance, particularly if associated with mealtimes.
Encourage a balanced diet meals on wheels, dietitian, etc.
Reduce smoking and alcohol intake as much as possible.
Clear, large labelling is essential.
Small tablets make swallowing easier as do liquid formulations.
Avoid modified-release (SR, LA) products unless they are pharmacologically justified. e.g. the short half-lives of nifedipine and diltiazem make them unsuitable for use except in the SR/LA formulation; note that
when prescribing maintenance treatment using any SR/LA formulation, the same brand name should always be used due to variations in pharmacokinetics between brands.
Avoid fixed-dose combinations unless they aid compliance.
Do not use NSAIDs for analgesia only.
Consider the individual’s biological age, not their chronological age.
Expect adverse drug reactions and interactions in the elderly.
Cooperate with the patient, the carer, the nurse and the pharmacist, to
foster compliance at every opportunity.
advises that patients on medication should have a medical review at least annually and 6-monthly if they are on more than four medications.
Written summary of changes and clear medication lists will improve patients’ understanding of their medicines.
SUGGESTIONS FOR RATIONAL GERIATRIC PRESCRIBING
Points to consider when prescribing for elderly patients What is the patient’s ‘biological age’, ie. is the patient fit for his or her age or should special care be taken with medication, due to overt organfailure?
Should a low starting dose be used (e.g. calcium-channel blockers, many
antidepressants, all benzodiazepines)?
Does this drug have a small margin of safety (e.g. digoxin, theophylline, lithium, warfarin)?
What is its route of elimination (e.g. avoid chlorpropamide and glibenclamide in any degree of renal impairment)?
What interactions may occur with the existing treatment?
Could the new drug worsen existing pathology (e.g. NSAIDs)?
Questions to consider when reviewing the current long-term drug treatment of elderly patients
Is the medication strictly necessary?
Is it being taken?
Are there any side-effects?
Is it having any therapeutic effect?
Are any of the drugs incompatible?
Are there signs of drug-drug interaction?
Drugs and renal impairment
hacking-medschool/drug-cautions-ckd
Drugs and the Liver
hepcnet.net drugs and liver damage
Phase 1 battery of cytochrome P450 oxidase iso-enzymes each acting on different group of drugs
Phase 2
Inhibition
Induction.
Drug interactions
- <a title="
Flockhart DA. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine 2007″ href=”http://medicine.iupui.edu/clinpharm/ddis/ClinicalTable.aspx”>P450 Drug Interactions Indiana University
- Drug Interaction Checker @ Drugs.com
- Drug Interaction Checker @ Medscape
- youtu.be/EJ37Pm1RtNg
- youtu.be/5ez7g5hNkoo
Drugs commonly involved in important interactions
ACE inhibitors
anti-epileptic drugs
antidepressants
anti-inflammatory drugs
amiodarone
beta-blockers
cimetidine
digoxin
diuretics
hypoglycaemic agents (antidiabetics)
lithium
oral contraceptives
rifampicin
theophylline
warfarin
Enzyme Inhibition
smoking
all imidazole antifungals watch for the suffix ‘-azole’ affect many other drugs and increases the plasma concentration of over 70 other drugs.
Cimetidine (Tagamet) One of the very few drugs that inhibit almost all C450 groups; it is available without prescription in the UK
Grapefruit juice increases the plasma concentration of over 40 other drugs. Tell patients on maintenance regimes not to drink grapefruit juice or eat grapefruit.
A 250-ml glassful knocks out a generation of P450 in the intestinal wall and it takes 24 hours to recover. More than 500 ml/day also knocks out liver CYP3A4
macrolides (erythromycin, etc.) increase the plasma concentration of over 25 other drugs
| drug interactions via Enzyme induction | |
|---|---|
| SSRIs | TCADs BZDs antipsychotics NSAIDS |
| verapamil diltiazem | |
| amiodarone | warfarin, digoxin and ciclosporin |
| metronidazole | including lithium and alcohol |
| quinolones (ciprofloxacin) | methotrexate and theophylline |
| omeprazole | diazepam and digoxin |
| allopurinol | ciclosporin |
| sulphonamides | phenytoin |
Enzyme Inducers increase the metabolism of other drugs with risk of treatment failure
9 liver enzyme inducers = four anti-epileptics, the rifamycins, St John’s wort, griseofulvin and two antivirals
- barbiturates
- primidone
- phenytoin
- carbamazepine
- rifamycins
- ST Johns Wort
- griseofulvin
- efavirenz
- nevirapine
Interaction between St John’s Wort and hormonal contraceptives
PolyPharmacy
Drug Interactions Hypertension & CCF
Avoid NSAIDS
In regimens for hypertension and congestive heart failure (CHF), NSAIDs will cause significant deterioration of control. There is a particular risk in prescribing an NSAID (or the patient taking an OTC NSAID) for any patient on an ACEI or ARB, as both drugs reduce renal function by around 20% and both cause hyperkalaemia. NSAIDs should
be avoided, or a different hypotensive chosen. In addition, any NSAID will reduce the hypotensive effect of any antihypertensive regimen through loading of extra Na+ and water, made worse by blocking the positive inotropic effect of physiological prostaglandins on the myocardium.
Beta-blockers are used in HF but can cause worsening of myocardial function
Drug interactions anti-arrythmics
May cause synergystic depression of both the cardiac conducting tissues and the general myocardium.
especially if two or more are co-prescribed. The other major risk is prescribing an anti-arrhythmic drug to any patient who is taking digoxin.
1 Amiodarone added to digoxin incurs a high risk of acute heart failure and asystole
2 The most powerful calcium-channel blockers diltiazem and verapamil may precipitate CHF if ventricular function is borderline.
Such drug combinations are best used only on specialist recommendation after echocardiogram assessment and with regular plasma electrolyte monitoring.
(Chapter 4 McGavcock)
Drug interactions impotence drugs
sildenafil, tadalafil and vardenafil.
These pose a great risk to all patients taking slow-release nitrates for angina. Their potent vasodilation is not confined to the penis, and can cause widespread systemic vasodilation, profound hypotension and collapse in a patient who is taking a slow-release nitrate.
Avoid grapefruit juice as they increase the bioavailability of the erection enhancers.
1 Do not use in hypotensive patients.
2 Do not use after a recent stroke.
3 Do not use within six months of a myocardial infarction.
4 Do not use in unstable angina.
S Take extra care when prescribing for patients with multiple myeloma or leukaemia.
6 Examine the penis for anatomical abnormality before prescribing.
7 Tell all angina patients who are taking slow-release nitrates to avoid erection enhancers, and record your warning in the patient’s records.
More interactions
Remember also that the interacting agent may be an OTC drug.
The combination of aspirin with an anti-inflammatory drug is the commonest cause of bleeding from the stomach.
Metoclopramide Ciclosporin: Metoclopramide increases plasma concentration of ciclosporin
Hydrocortisone Carbamazepine accelerates metabolism of corticosteroids (reduced effect)
Prochlorperazine/haloperidol Amiodarone: increased risk of ventricular arrhythmias when phenothiazines given with amiodarone —avoid concomitant use
Moxifloxacin: increased risk of ventricular arrhythmias when phenothiazines given with moxifloxacin —avoid concomitant use
Phenytoin: antipsychotics antagonise anticonvulsant effect of phenytoin (convulsive threshold lowered)
Sibutramine: increased risk of CNS toxicity when antipsychotics given with sibutramine (manufacturer of sibutramine advises avoid concomitant use)
Sotalol increased risk of ventricular arrhythmias when phenothiazines given with sotalol
Valproate antipsychotics antagonise anticonvulsant effect of valproate (convulsive threshold lowered)
Increased risk of ventricular arrhythmias when anti-arrhythmics that prolong the QT interval given with antipsychotics that prolong the QT interval
Tramadol
Coumarins: tramadol enhances anticoagulant effect of coumarins
MAOIs: possible CNS excitation or depression (hypertension or hypotension) when opioid analgesics given with MAOIs —avoid concomitant use & for 2 weeks afterstopping MAOIs
Moclobemide: possible CNS excitation or depression (hypertension or hypotension) when moclobemide given with opioid analgesics
SSRIs: increased risk of CNS toxicity when tramadol given with SSRIs
Tricyclics increased risk of CNS toxicity when tramadol given with tricyclics
Ciprofloxacin Ciclosporin increased risk of nephrotoxicity when quinolones given with ciclosporin
Coumarins: ciprofloxacin enhances anticoagulant effect of coumarins
Duloxetine: ciprofloxacin inhibits metabolism of duloxetine —avoid concomitant use
ciprofloxacin increases plasma concentration of theophylline
NSAIDs: possible increased risk of convulsions when quinolones given with NSAIDs
Codeine Moclobemide: possible CNS excitation or depression (hypertension or hypotension) when moclobemide given with opioid analgesics
Doxycycline
Ciclosporin: doxycycline possibly increases plasma concentration of ciclosporin
Coumarins: tetracyclines possibly enhance anticoagulant effect of coumarins
Erythromycin
Amiodarone: increased risk of ventricular arrhythmias when parenteral erythromycin given with amiodarone —avoid concomitant use
Carbamazepine: erythromycin increases plasma concentration of carbamazepine
Ciclosporin: erythromycin inhibits metabolism of ciclosporin (increased plasma
concentration)
Clozapine: erythromycin possibly increases plasma concentration of clozapine
(possible increased risk of convulsions)
Colchicine: erythromycin increases risk of colchicine toxicity
Coumarins: erythromycin enhances anticoagulant effect of coumarins
Ivabradine: increased risk of ventricular arrhythmias when erythromycin given with ivabradine
Midazolam: erythromycin inhibits metabolism of midazolam (increased plasma concentration with increased sedation)
Reboxetine: avoidance of macrolides advised by manufacturer of reboxetine.
Sertindole: increased risk of ventricular arrhythmias when erythromycin given with
sertindole —avoid concomitant use
Simvastatin: increased risk of myopathy when erythromycin given with simvastatin (avoid concomitant use)
Theophylline: erythromycin inhibits metabolism of theophylline (increased plasma concentration), if erythromycin given by mouth, also decreased plasma erythromycin
concentration
Verapamil: erythromycin possibly inhibits metabolism of verapamil (increased risk of toxicity)
Ibuprofen
Coumarins: ibuprofen possibly enhances anticoagulant effect of coumarins
Methotrexate: ibuprofen reduces excretion of methotrexate (increased risk of toxicity)—but for concomitant use in rheumatic disease see Methotrexate, section
SSRIs: increased risk of bleeding when NSAIDs given with SSRIs avoid concomitant use of NSAIDs with aspirin (increased side-effects)
Ciclosporin: increased risk of nephrotoxicity when NSAIDs given with ciclosporin
Phenytoin: NSAIDs possibly enhance effects of phenytoin
Quinolones: possible increased risk of convulsions when NSAIDs given with quinolones
Sulphonylureas: NSAIDs possibly enhance effects of sulphonylureas
Prednisolone
Carbamazepine accelerates metabolism of corticosteroids (reduced effect) Grapefruit Juice Contains furanocoumarol,which irreversibly blocks p450 Cytochrome oxidase drug/toxin metabolising system in the small intestine.
Drugs whose normal recommended dosage regime takes this into account will be absorbed in higher concentrations (up to double) for the next 24 hours (time it takes the enzymes to be regenerated)
One 250ml glass Grapefruit juice or half a grapefruit or 1 Seville Orange is enough.
Risks increased in elderly and those with reduced renal functioning
Calcium-blockers all except diltiazem Hypotension with all. Risk of heart block and heart failure as well, with verapamil
amiodarone as above plus risk of circulatory collapse and hepatic toxicity
simvastatin and atorvastatin Increased risk of liver toxicity and sideeffects
All erection-enhancing drugs Hypotension, priapism and other side-effects
Immunosuppressants
ciclosporin, sirolimus tacrolimus Grafted rejection
Antivirals efavirenz and saquinavir Treatment Failure
warfarin Enhanced anti-coagulation and its risks
buspirone Excessive and prolonged sedation
carbamazepine Risk of blood, liver and skin disorders
sertraline Hypotension, tachycardia, confusion, amnesia, aggression and serious liver and pancreatic disorders
Pitfalls In Prescribing & How To Avoid Them Hugh McGavock 2009 Oxford Radcliffe Antacid Interactions & Treatment Failure Drugs whose absorption is impaired if an antacid has been taken
ACE inhibitors Poor control of hypertension
Antibiotics: azithromycin ciprofloxacin
isoniazid, rifampicin most tetracyclines Infection does not respond to treatment
antivirals, amprenavir, atazanavir,tipranavir Infection does not respond to treatment
antifungals itraconazole and ketoconazole Infection does not respond to treatment
anti-epileptics phenytoin and gabapentin Risk of seizures
phenothiazine antipsychotics and sulpiride Recurrence of psychotic symptoms
anti-malariaIs: chloroquine,hydroxychloroquine and proguanil Failure of anti-malarial protection
bisphosphonates Failure of osteoporotic therapy
digoxin Treatment failure
oral iron Anaemia fails to respond
lansoprazole Failure of ulcer or GORD treatment
antihistamine fexofenadine Symptoms unrelieved
dipyridamole Reduction in prophylactic effect
rosuvastatin Failure of lipid lowering
Always ask re concomittant OTC antacid use which is very common.
Advise take after meal if must be taken whilst drug taken before meal
1 formation of a chemical complex complexation
2 adsorption of the drug by the antacid
3 resin binding
4 destruction of a drug’s acid-resistant coating due to the increased pH in the stomach that results from the antacid.
Pitfalls In Prescribing & How To Avoid Them Hugh McGavock 2009 Oxford Radcliffe
Alcohol interactions
1 Occasional drinking inhibits members of the P450 enzyme group, reducing the metabolism of many drugs and leading to an increase in their plasma concentration.
2 Conversely, chronic, regular heavy drinking induces the same enzymes, increasing drug metabolism and leading to a reduced plasma drug concentration and therapeutic failure – particularly affected are
warfarin
tolbutamide
doxycycline
antidepressants
antipsychotics
the benzodiazepines
paracetamol
ANTABUSE-LiKE REACTIONS
vomiting, flushing, headache, palpitations and (if the alcohol intake is large), hypotension and collapse
cephalosporins
metronidazole
sulfonamides
isoniazid
griseofulvin
sulphonylureas
nitrofurantoin
nitrates
McGavock
Cranberry juice
Enhances the anticoagulant effect of warfarin (and other coumarins) in some patients
Information for patients taking long-term steroids
Steroid card
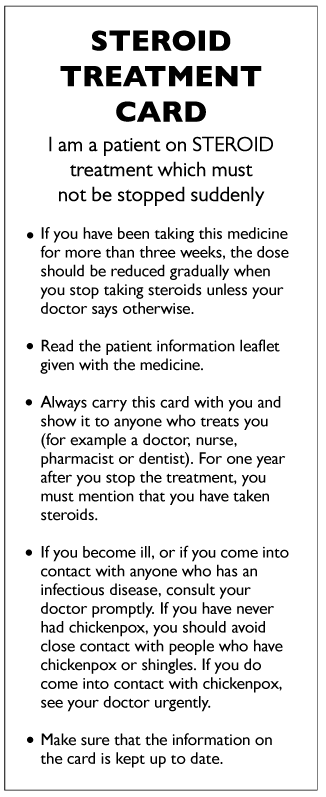
Preventing steroid induced osteoporosis
Consider every patient for active osteoporosis prevention who has had:
Oral or iv steroid treatment for greater than 3 months.
A cumulative lifetime dose of 1g oral prednisolone.
Inhaled steroid > 1000mcg day beclomethasone.
If unable to have a DEXA then treat, 1st line = a bisphosphonate.
If steroid course likely to > 3 months then treat, 1st line = a bisphosphonate.
If able to have a DEXA and the T score above 1.5 treat with lifestyle measures +/ calcium supplementation.
NB DEXA needs repeating every 3 years
If the T score is = 1.5 or lower then treat, 1st line = a bisphosphonate.
NB DEXA needs repeating every 3 years
Bisphosphonates – e.g. Alendronate (Fosamax) – 5mg daily for primary prevention (e.g. in patients on long term oral steroids deemed to be at risk), 10 mg a day or 70mg once weekly for secondary prevention i.e. proven osteoporosis or patients with osteoporotic vertebral collapse or low velocity fractures. In large randomised controlled trials, the bisphosphonate alendronate reduced both vertebral and nonvertebral fractures. It is most beneficial in those at highest risk–women with at least one previous vertebral fracture or osteoporosis. Symptomatic vertebral fractures were decreased by 2836% over four years’ treatment, whereas the risk of hip fracture was reduced by just over a half.
There are now monthly oral bisphosphonates and 3 monthly iv bisphosphonates for those patients who tolerate them poorly.
Advice for taking Fosamax to reduce GI side effects = Prior to breakfast, after getting up swallow the tablet with a drink of water and then no food for 30 minutes and no lying down until after eating and report any dyspepsia or dysphagia symptoms to your GP. (Remember two important contraindication to bisphosphonates are a history of achalasia or a history of oesophageal stricture).
Adverse drug reactions (ADRs)
ADR reporting
Report all ADRs including self-medication and herbal products especially:
any reaction from new drugs (marked with black triangle in the BNF T in MIMS)
severe reactions from established drugs even if a well recognised reaction
children
Adverse drug reaction reporting
Report all serious suspected reactions to established drugs and report all suspected reactions (including those considered not to be serious) to drugs showing the black triangle symbol
An adverse drug reaction (ADR) is an unwanted or harmful reaction experienced following the administration of a drug or combination of drugs under normal conditions of use, which is suspected to be related to the drug. The reaction may be a known side effect of the drug or it may be new and previously unrecognised. Rapid detection and recording of adverse drug reactions is vital so that hazards are identified promptly and appropriate regulatory action is taken to ensure that medicines are used safely. Suspected ADRs to any therapeutic agent should be reported, including drugs (self-medication as well as those prescribed), blood products, vaccines, radiographic contrast media, complementary and herbal products.
Established drugs
Healthcare professionals are asked to report all serious suspected reactions to established drugs (including over-the-counter, herbal, and unlicensed medicines and medicines used off-label) and vaccines. Serious reactions include those that are:
* fatal
* life-threatening
* disabling or incapacitating
* have resulted in or prolonged hospitalisation
* congenital abnormalities
* medically significant
They should be reported even if the effect is well recognised. A severe reaction might not be life-threatening or disabling but can seriously affect an individual patient. For example, headaches are not normally considered serious in nature, but may be very severe. A severe or exaggerated ADR should be reported.
Newer drugs
A Black Triangle is assigned to a product if the drug is an active substance which has been newly licensed for use in the UK.
However, a product containing previously licensed active substances may also be monitored and assigned Black Triangle status if it meets one or more of the following criteria:
* it contains a new combination of active substances
* administration of the drug via a new route of administration or drug delivery system
* a significant new indication which may alter the established risk/benefit profile of that drug
* an established medicine which is to be used in a new patient population
All similar biological medicines (biosimilars) have a Black Triangle symbol because every new biological product has been developed to be similar to an existing biological product, however may not have an identical structure therefore requires intensive monitoring for safety and efficacy. Healthcare professionals are asked to report all suspected adverse reactions (including those considered not to be serious) associated with Black Triangle products, in order to:
* confirm risk/benefit profiles established during the pre-marketing phase
* increase understanding of the safety profiles of new medicines
* ensure that previously unrecognised side effects are identified as quickly as possible
- Drug Induced Pulmonary Toxicity Medscape
- Drug-Induced Lung Disorders US pharmacist
- Drug-Induced Lung Disease ajronline
Easily missed. Diagnosis may be overlooked / delayed causing permanent morbidity / mortality (Mcgavock)
| ADRs affecting the lung | |
|---|---|
| Asthma | beta-blockers (inc eye drops)aspirinNon-steroidal anti-inflammatory drugsAny nebulised medication |
| Chronic cough | Angiotensin-converting enzyme inhibitors |
| Interstitial pneumonitis/fibrosis | amiodaronenitrofurantoinbleomycinbusulfan
cyclophosphamide methysergide phenytoin |
| Acute hypersensitivity pneumonitis | infliximab (anti-TNFa)interferon a and leflunomide |
| Pulmonary oedema | non-cardiogenicaspirinchlordiazepoxidecocaine, especially inhaled ‘crack’
heroinCardiogenic beta-blockers clomiphene |
| Pleural effusion | bromocriptinenitrofurantoinAny drug which induces systemic lupuserythematosus
methysergide Chemotherapeutic agents |
| Mediastinal widening | phenytoincorticosteroidsmethotrexate‘crack’ cocaine |
| Pulmonary infiltration | Without eosinophiliaamitriptylineazathioprineamiodarone
crack’ cocaine (inhaled)With eosinophilia sulfonamides L-tryptophan nitrofurantoin penicillins methotrexate |
ADRs high risk drugs medication errors
- ADRs AAFP 2003
- High Risk Drugs NPC Jul 2011
- Prevalence of adverse drug events in ambulatory care
- Drug-related problems causing hospitalisation
Adverse drug reactions account for about 20% of acute hospital admissions and occur in 10-20% of hospital inpatients.
Type A reactions are those which can be predicted from the known action of the drug
Type B reactions are unpredictable and unrelated to the dose administered
there are two main causes of type A reactions decreased removal by the liver and kidney and excessive sensitivity to the action of the drug.
For example, the slow metabolism (breakIng down) of morphine by the liver in patients with liver damage can cause excessive sedation and even coma. Impaired elimination of digoxin by the kidney can result in anorexia, nausea, heart irregularities and disturbance of colour vision. Increased sensitivity to the action of digoxin can also occur in patients with a low potassium concentration in the
blood, and patients with chest disease are more susceptible to the depressant effect of morphine on respiration.
Type B reactions are unusual and unexpected. There are a number of factors which make the patIent more likely to develop these adverse effects.
Certain enzymes (chemicals which convert one substance to another) are absent from red blood cells of Afro-Caribbeans) making them more susceptible to haemolysis (breakdown of red cells) when a number of drugs (e.g. antimalarials) painkillers) antimicrobials) are administered. Environmental factors diet) pollution) intake of alcohol, tobacco and otherrecreational drugs are also probably important) but these have not been studied in sufficient detail to allow accurate predictions.
csiro.au/nid/270/paper/AH09863
Allergic drug reactions
??? McGavock
Initial exposure results in the formation of antibodies which attach to the surface of mast cells. The mast cells are destroyed, releasing a number of substances such as histamine and prostaglandins which make blood vessels dilate and so reduce the blood pressure. This can result in allergic rashes, but the most severe reaction is anaphylaxis.
- Other examples of allergic reactions
- include haemolysis
- depression of the bone marrow
- jaundice
- kidney damage
- ADrs BMJ
- world allergy drug allergy
- aaaai drug allergy
- Cleveland clinicdrug allergies
- drug allergies UMMC
- allergy clinic.co.uk medication allergy
- Study suggests that around one quarter of suspected cutaneous drug reactions are not actually due to medicines NeLM BJD Jan 2012
QTc
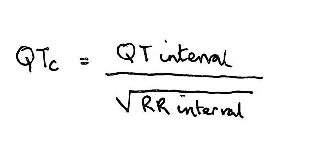
- QTc Prolongation and Risk of Sudden Cardiac Death: Is the Debate Over? Medscape 2006
- QTc mdcalc.com
- Long QT @ Arizona CERT
Normal QTc < 440 msec. A longer QTc puts the patient at increased risk for fatal ventricular arrythmia torsade de pointes, particularly when prescribed drugs which themselves cause QT prolongation.
Risk is multiplied by co-prescribing of other drugs causing and/or coexistance of one or more of these conditions
Causes
- Congenital Long QT Syndrome
- CAD, Cardiomyopathy, AF
- Severe Bradycardia, High-Grade AV Block
- HypoCa, HypoK HypoMg other lyte abnormalities
- Autonomic dysfunction
- Hypothyroid
- Hypothermia
Drugs
- Anti-Arrhythmics – amiodarone sotalol disopyramide procainamide
- Antibacterial erythromycin clarithromycin moxifloxacin pentamidine
- Calcium blockers diltiazem verapamil
- Antipsychotics All of the older (phenothiazine) drugs haloperidol pimozide sertindole amisulpride
- Antidepressants All tricyclics, particularly dosulepin (Prothiaden), fluvoxamine paroxetine lithium
- Anti-emetics domperidone
- Anti-fungals ketoconazole
- Antihistamines mizolastine
- Analgesics methadone
Therapeutic drug monitoring
Drug Safety Monitoring BetterTesting
| Drug | Timing | Range | when to test |
|---|---|---|---|
| Digoxin | 6 – 12 hrs after last dose | 0.8 – 2 mcg/l1-2 nmol/l | if toxicity suspected |
| Lithium | 12 hours after last dose | 0.5-1.5 mmol/l | 3 monthly when stable |
| Phenytoin | just before next dose (ie trough) | 10-20 mg/l40-50 micromol/l | for dose adjustment |
| Theophylline | |||
| Procainemide | |||
| NHS Tayside Nov 2007 / Pastest 2006 / GPCSG | |||
Some drugs need to be monitored at the “steady state” ie 4-5 half lives after initiation of therapy or a change in dosage. Care needs to be taken with loading doses eg digoxin and phenytoin where misleading concentrations may occur in the early stages of treatment if the timing of specimens is wrong.
Failure to meet these requirements accounts for most errors in therapeutic drug monitoring.
Drugs for which the laboratories provide a routine monitoring service are listed in the table below together with details of specimen tubes and collecting times. It is possible to monitor other drugs and special arrangements can be made with the Department of Biochemical Medicine at Ninewells Hospital for certain pharmaceuticals. Throughout the Formulary and Antibiotic Policy, an indication is given when therapeutic monitoring is available for a listed drug. It is not essential that this is carried out in all cases but may be considered during the early treatment period if complications arise. Further information on laboratory services for therapeutic drug monitoring can be obtained from Departments of Biochemical Medicine and Medical Microbiology and further advice may be obtained from the clinical pharmacist at ward level or from the Medicines Information Service extn 32351.
DRUG SPECIMEN COLLECTION TIME THERAPEUTIC INTERVAL TIME TO STEADY STATE (days)
Carbamazepine SST Pre-dose 4-10 milligram/L 5
Ciclosporin EDTA Pre-dose Therapeutic range depends on clinical situation (see below) 5
Digoxin* SST Pre-dose or at least 6 hours after the last dose 1.0 – 2.0 microgram/L 7
Lithium SST 12 hours after the evening dose 0.4 – 0.8 millimol/L (prophylaxis)
0.8 – 1.2 millimol/L (mania) 4
Methotrexate SST 24 and 48 hours post infusion under 1 micromol/L at 48 hours –
Phenobarbital** SST Anytime 10-40 milligram/L 20
Phenytoin*** SST Anytime 10-20 milligram/L Variable about 14 days
Tacrolimus EDTA Pre-dose 5 – 15 microgram/L 3
Theophylline SST Peak levels – 6 hours after sustained release dose. Trough levels – pre-dose <4 years: 5-10 milligraml/L
>4 years: 10-20 milligraml/L 2
* Potassium levels should be measured at the same time as diogoxin since this can alter the interpretation of the results
** Also used for monitoring primidone
*** Following fosphenytoin administration, there should be an interval of 4 hours before blood specimens are collected
Drugs needing monitoring
ACE inhibitors
Amiodarone
Azathioprine
Carbimazole
Propylthiouracil
Clozapine
Cyclophosphamide
Digoxin
Diuretics
Erythropoietin
Gold
Methotrexate
Penicillamine
Statins
Sulphasalazine
Vitamin D
Warfarin
1 pretreatment tests to establish a baseline
2 the desirable therapeutic range (where appropriate)
3 the frequency of test repetition for therapeutic range
4 tests during maintenance therapy to detect known adverse effects of the drug regimens
5 the frequency of test repetition
6 further special monitoring.
| Specific Drugs | |
|---|---|
| Warfarin |
Wisconsin Alumni Research Foundation Vitamin K antagonist anticoagulant with narrow therapeutic range and important drug interactions prevention of deep vein thrombosis (DVT), pulmonary embolism, embolisation in atrial fibrillation and some prosthetic heart valves. It is also used during haemodialysis and to prevent myocardial infarction in patients with unstable angina Warfarin and NSAIDs High risk of profuse gastrointestinal bleeding Warfarin and macrolides(e.g. erythromycin) Potentiation of warfarin Warfarin and quinolones(e.g. ciprofloxacin) Potentiation of warfarin Warfarin and phenytoin Potentiation of effects of warfarin and phenytoin Warfarin and amiodarone Potentiation of warfarin NSAID MACRO QUINO PHENO AMIO Warfarin Colours – i fact depends on preparation 0.5 mg white 1 mg 3 mg blue 5 mg Add this information to patients prescription and reinforce verbally “one blue plus one white” Check INR regularly and at the same time (warfarin dosage traditionally 6pm with INR in morning on wards) INR therapeutic Ranges
Warfarin reversal |
| Methotrexate | |
| Lithium |
Patients are still being harmed and killed because they have not had their dosage adjusted based on recommended regular blood tests. The Community Mental Health Teams and elderly care teams will ensure that lithium levels are taken every three months and that these results are communicated to the GP to be recorded on the clinical system. They will also inform patients on lithium that their levels will be shared with the health professional involved in their lithium treatment (including community pharmacists) and obtain patient consent for these levels to be shared. The NPSA has developed a patient information booklet, lithium alert card and record book for tracking blood tests. This is similar to the warfarin booklets and is purple in colour. The responsibility for the supply of patients’ booklets, lithium alert cards and record books lies with the healthcare practitioner who initiates the therapy. In most cases this will be the consultant psychiatrist. Some spare copies are also available from the medicines management team. Community pharmacists need to ensure they have access to a recent level to allow them to dispense safely and so may be contacting GP practices to obtain levels if the patient does not have their Lithium booklet with them, or it has not been updated. Please facilitate the safe dispensing of lithium by providing them with up to date levels as required. Currently in Salford the majority of patients are monitored by CMHTs and the lithium prescribed by the GP. To ensure safe prescribing practice in these patients a Shared care protocol (SCP) has been written by Greater Manchester West Foundation Trust which highlights the monitoring required, and gives useful information on prescribing lithium safely. When new patients are initiated on lithium from by secondary care, a signed SCP will be sent to the GP practice. If you identify patients who have not had a recent lithium level (a audit was carried out in Novemberand so hopefully this should be minimal), do not stop the prescribing of lithium as abrupt cessation of therapy can be harmful, but liaise with the patients CMHT or elderly care service to determine when a level will be taken and the plans in place to obtain this. Discontinuation of lithium therapy should only be considered after discussion with the consultant in charge of the patient’s care. There are a small number of patients that have been stepped down from secondary care and the GP practice carries out the lithium monitoring. Practices need to identify if they have any patients they monitor and ensure that three monthly monitoring of Lithium and six monthly monitoring of TFTs and U and Es is occurring. The SCP can be used as a guide to good practice, but patients do not need entered into it. These patients will also have to obtain their lithium booklets from the GP practice. Contact the medicines management team to obtain one. Please note lithium therapy is not currently in the near patient testing LES but more frequent testing has been suggested by NICE as an indicator for 2011/2012 QOF. |
| Amiodarone | Antiarrythmic for atrial and ventricular tachyarrythmias. Toxic in its own right and subject to important interactions.Initiate under specialist supervision only. |
| NSAIDS |
MHRA Sep 2011 Hull York Medical School has led an international review which reports that the use of popular non-steroidal anti-inflammatory drugs, such as diclofenac can increase the risk of heart attack or stroke by a third. Non-steroidal anti-inflammatory drugs (NSAIDs) form an extremely important and widely-used group of medicines, for the treatment of arthritis and many other painful conditions, including headache, fever, and minor ailments. For most patients the risks of side effects are outweighed by the benefits of treatment. The findings of this study are not new; an increase in risk of heart attack and stroke with some NSAIDs has been well recognised for some years, particularly with long-term use of high doses and in patients who are already at high risk. The safety profile of all NSAIDs, and in particular their possible association with adverse cardiovascular effects, such as heart attack or stroke, has been carefully evaluated by the MHRA, on many occasions, as new data has become available. Clear information about the risk of heart problems, along with information about those patient groups in which NSAIDs either should not be used (such as those with severe heart failure) or used with caution, are contained in the information for healthcare professionals and the leaflet for patients that accompanies the medicine. To minimise the risk of side effects, our advice remains that all NSAIDs should be used for the shortest time and at the lowest dose necessary to control symptoms. People should not stop taking their NSAID medicine, but if they have any questions about their treatment they should speak to their doctor or pharmacist. |
| PPIs | PPI safety |
| Antidepressants and antipsychotics | |
| Prescribing antibiotics | hacking-medschoolantibiotic-guidelines |
| 10 | x |
| 11 | x |
Dabigatran (pradaxa)
NOACS
two new oral anticoagulants for stroke prevention in people with AF
dabigatran a direct thrombin inhibitor
rivaroxaban, a factor Xa inhibitor
NOACs should only be considered as an alternative to warfarin for stroke prevention in AF in patients who are:
• Unable to take warfarin due to allergy or contraindications
• Unable to adhere to the monitoring requirements associated with warfarin therapy
• Unable to achieve an INR within the target therapeutic range (TTR) for a satisfactory period of time after a suitable trial of warfarin.
Dabigatran received a positive opinion by the European Medicines Agency on 14.4.11 for
“Prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation with one or more of the following risk factors:
Previous stroke, transient ischemic attack, or systemic embolism
Left ventricular ejection fraction < 40 %
Symptomatic heart failure, ? New York Heart Association (NYHA) Class 2
Age ? 75 years
Age ? 65 years associated with one of the following: diabetes mellitus, coronary artery disease, or hypertension
Greater Manchester Medicines Management Group (GMMMG) and the Greater Manchester and Cheshire Cardiac and Stroke network (GMCCSN) are currently reviewing the use of Dabigatran and how it is positioned in management pathways as anticoagulant services will need to be redesigned to modateaccom the use of this new drug. This needs to happen before wide spread use of the drug otherwise we will be paying for both anticoagulation clinics which we might not require and having to absorb the cost of Dabigatran
Shared care drugs
Red Amber Green List
Red drugs should not be prescribed in primary care eg LMWH and erythropoietin.
If asked to prescribe or administer an unfamiliar or dubious drug by secondary care check with PCO prescribing guidelines.
http://www.nyrdtc.nhs.uk/GMMMG/Groups/ipnts/ipnts_rag/ipnts_rag.php
Bucks Shared Care Protocols and Amber Initiation Guidance Jun 2011 @ NeLM
Legal responsibility for prescribing lies with the doctor who signs the prescription.
GPs often have to issue repeat prescriptions between hospital reviews so must ensure that there is written documentation and test results available from the hospital visits in the medical record before prescribing.
Chasing up such information can be timeconsuming, but is essential for patient safety and can be delegated to a member of staff.
This is particularly important for drugs such as methotrexate and cyclosporin. Your PCO and hospital trust may have a ‘shared care agreement’ which sets out clear guidance for patients under shared care.
Medication changes should be recorded in the prescribing and medical record, and review dates amended when hospital letters are dealt with. Enter the results of any drug monitoring tests to prevent duplication of these.
record, and review dates amended when hospital letters are dealt with . Enter the results of any drug monitoring tests to prevent duplication of these.
Medication Review To ensure safe prescribing for the many patients on longterm treatments, systematic reviews of medication and a foolproof repeat prescribing system are essential.
Red List – high cost / high risk meds
Some expensive specialist drugs are initiated in secondary care and GPs are then requested to continue prescribing them.
GPs are not obliged to prescribe if they feel insufficiently experienced or are not willing to accept the clinical responsibility involved. This is particularly relevant for new specialist treatments.
Such treatments, which include fertility drugs, erythropoetin injections and enteral nutritional supplements, may cost thousands of pounds per patient per year.
Your practice should have a policy on such prescribing based on locally approved shared care guidelines.
As a new GP it should not be up to you to decide whether or not to prescribe such drugs for patients.
GPs asked to prescribe high cost medicines may need to seek advice from the prescribing advisor for their pca as sometimes these items can be accounted for in the annual practice drug costs.
Drug expiry dates
Drug expiry dates
Prescribing regs
Pharmaceutical Services Negotiating Comittee
Medicines for human use © Royal Pharmaceutical Society of Great Britain 2003
3 classes of products under the Medicines Act 1968
(1) General sale list medicines (GSL)
(2) Pharmacy medicines (P)
(3) Prescriptiononly medicines (POM).
General sale list medicines (GSL)
All general sale list medicines, except those that have been designated as foods or cosmetics, must be licensed products (it should be noted that a medicinal product made up in a pharmacy for sale from that pharmacy without a marketing authorisation, is classified as a pharmacy medicine even though all its ingredients are in the GSL Order).
Products not on general sale
Part of the GSL order specifies certain classes of medicinal products for human use which shall not be available on general sale. They are medicinal products promoted, recommended or marketed:
(a) for use as anthelmintics,
(b) for parenteral administration,
(c) for use as eye drops,
(d) for use as eye ointments,
(e) for use as enemas,
(f) for use wholly or mainly for irrigation of wounds or of the bladder, vagina or rectum.
(g) for adminstration wholly or mainly to children being a preparation of aloxiprin or aspirin.
Retail sale of GSL medicines
Medicinal products on a general sale list may only be sold by retail, offered or exposed for sale by retail, or supplied in circumstances
corresponding to retail sale either at registered pharmacies, or in circumstances where the following conditions are fulfilled:
(a) The place at which the medicinal product is sold, offered, exposed for sale or supplied, must be premises at which the person carrying on the business is the occupier and which he is able to close so as to exclude the public. Sales from automatic machines should only be made from machines located in premises which the occupier is able to close so as to exclude the public.
(b) The medicinal product must have been made up for sale in a container elsewhere and not have been opened since the product was made up for sale in it.
Pharmacy Only (PO)
A PO medicine is a product that is licensed as a GSL medicine, but is restricted to sale through pharmacies only. PO medicines do not need to be sold under the supervisison of a pharmacist, however the premises must be under the personal control of a pharmacist which is a requirement for pharmacy premises to be lawfully trading. These medicines may be available for self-selection by members of the public.
1.2.2 Pharmacy medicines (P)
A pharmacy medicine means a medicinal product which is not a prescription only medicine and which is either:
(a) not a medicinal product on a general sale list, or
(b) a product referred to in Regulation 8 of the Medicines (Sale or Supply) (Miscellaneous Provisions) Regulations 1980.
The products referred to in Regulation 8 are:
(a) Products for human use containing aloxiprin, aspirin, paracetamol or salicylamide which are offered or exposed for sale by retail in packs containing:
(i) in the case of effervescent tablets, (a) which do not contain aspirin, or where the amount of aspirin in each tablet does not exceed 325mg, more than 30 tablets;
(b)
where the amount of aspirin in each tablet exceeds 325mg, but does not exceed 500mg, more than 20 tablets;
(ii) in the case of tablets that are not effervescent, more than 16 tablets;
(iii) in the case of powder or granules, more than 10 sachets; and
(iv) in the case of capsules, more than 16 capsules.
(v) in the case of liquid preparations of paracetamol, intended for persons aged 12 years and over, more than 160ml; intended for persons less than 12 years, individual doses of more than 5ml each, or more than 20 unit doses.
(b) Tablets for human use containing bisacodyl which are offered or exposed for sale by retail in containers or packages containing more than 20 tablets.
(c) Products for human use containing ibuprofen which are offered or exposed for sale by retail in containers or packages containing:
(i) in the case of tablets, more than 16 tablets;
(ii) in the case of capsules, more than 16 capsules;
(iii) in the case of powders or granules, more than 12 sachets;
(iv) in the case of a product for topical use, more than 2.5g of ibuprofen.
(d) Products for topical human use containing clotrimazole which are offered or exposed for sale by retail in containers or packages containing more than 500mg of clotrimazole.
(e) Products for human use containing sodium picosulphate in a container or package of more than 60ml of the product.
(f) Products for human use containing loperamide hydrochloride in a container or package of more than 6 tablets or capsules.
Retail sale of pharmacy medicines
Pharmacy medicines may not be sold, offered or exposed for sale by retail, or supplied in circumstances corresponding to retail sale in the course of a business carried on by any person, unless:
(a) that person is, in respect of that business, a person lawfully conducting a retail pharmacy business;
(b) the product is sold, offered or exposed for sale, or supplied on premises which are a registered pharmacy; and
(c) that person, or, if the transaction is carried out on his behalf by another person, then that other person is, or acts under the supervision of, a pharmacist.
A retail pharmacy business must be under the personal control of a pharmacist so far as it concerns the sale of all medicinal products, including products on a general sale list.
The products referred to in the previous paragraph, ie, large packs of products containing aspirin, aloxiprin, paracetamol and salicylamide are not subject to the legal requirement for pharmacy medicines in that they need not be sold by or under the supervision of a pharmacist; however there is a professionalrequirement that these large packs be supervised.
© Royal Pharmaceutical Society of Great Britain 2003
FP10 (FP10 Comp)
Valid for 26 weeks from issue (CDs 13 weeks)
If you fill in number of days treatment, e.g. 30, then, providing you indicate the dosage and frequency of use, the pharmacist will dispense the precise quantity of drug. If not then you must specify the quantity of each drug to be dispensed along with dose and frequency
FP10s are issued by the Prescription Pricing Authority and bear the GP principal’s name, prescribing number, surgery address and telephone number so all prescriptions can be traced.
Named GPs, including all principals, have a prescribing number and should sign their own allocated prescriptions whereas registrars and some nonprincipals prescribe under their trainer’s or another principal’s name, but still maintain responsibility for each script they sign. If your name is not on the FPIO add it in block capitals to your signature so the pharmacist can identify you as the prescriber should any queries arise.
Accurate completion of prescriptions
Use indelible ink
Patient’s full name and address, plus age (if 12 years or under) or date of birth
Medicine name
Formulation (tablet, capsule, soluble, syrup, suppository, cream, patch)
Route (p.o., p.r., p.v., sublingual, buccal, topical, transdermal)
Dose – write out micrograms or nanograms in full, avoid the use of decimals and when used always ensure a 0 goes before the dot i.e. 0.5 ml not .5 ml
Frequency
Total quantity or duration of course
Signature (and print your name if not your prescription)
Date
o.d. /b.d./t.d.s. /q.d.s.
p.r.n. =as required (better give more specific instructions)
o.p. =standard pack size or tube of cream
You have legal and clinical responsibility for any prescription you sign.
Do not sign any you are not entirely confident about, particularly if they are initiated or generated by someone else
GOOD PRESCRIBING PRACTICE
You must always aim to adhere to good prescribing practice
Medication errors account for a large number of hospital admissions, complaints, and medicolegal claims. Try to be sure yoUR patient understands what they are being prescribed, what side effects to look out for and what adverse effects to report.
Give patients a list of their current medication.
Good prescribing safe prescribing
- Reporting adverse drug reactions Internal Link
- youtu.be/st5ioCqqn-4youtu.be/mLuR9-yMx8c
- youtu.be/LmuDJkODjuY
- youtu.be/ZSalY4Aeh1c
- MPS Factsheet Safe Prescribing
- NPC Ten Tips for Safer Prescribing June 2011
- Kings Fund Report on Quality of GP Prescribing Aug 2011
- Principles of conservative prescribing Arc Int Med Sept 2011
- RCP Sep 2011 N=1 N=1 Why people matter in medicines
- Avery et al BJGP AUG 2011 Development of prescribing safety indicators using RAND
- Continuity of Care – Keeping patients safe when they transfer between care providers – getting the medicines right NPC Rapid Review
- Prescribing Dilemmas: A Guide for Prescribers AWMSG
- eprescribing hazards
- http://www.ehi.co.uk/news/EHI/7256/research-finds-gp-e-prescribing-errors
- MPS tips for Safer Prescribing Oct 2011
- eTP
Consider carefully whether the treatment is really indicated
Always carry and consult with a current BNF
Prescribe safely check for: allergies interactions pregnancy and breast feeding
Prescribe generically unless medically inappropriate
Avoid combination formulations (except oral contraception and HRT)
Abide by existing practice formularies or prescribing protocols
Be aware of maximum duration of treatment per prescription
Limit the amount of drug you issue for known drug misusers or where you doubt the genuineness of request
Document all treatment fully in the medical record with review arrangements
Document medication reviews
Update prescribing records following home visits, OOH contacts and specialist reviews
Develop an awareness of drug costs (but remember cheaper is not always better)
Aim for evidencebased, costeffective and safe prescribing with a fully counselled patient. Keep up with the latest evidencebased guidelines, use drug information sources and develop a good relationship with your community pharmacist and PCO prescribing team.
Medication errors account for a large number of hospital admissions, complaints, and medico-legal claims
Try to be sure your patient understands what they are being prescribed, what side effects to look out for and what adverse effects to report. It is useful to give patients a list of their current medication.
Prescribe generically unless medically inappropriate
Avoid combination formulations (except oral contraception and HRT)
Abide by existing practice formularies or prescribing protocols – develop good relationships with local MMT.
Be aware of maximum duration of treatment per prescription
Limit the amount of drug you issue for known drug misusers or where you doubt the genuineness of request
Document all treatment fully in the medical record with review arrangements
Document medication reviews
Update prescribing records following home visits, OOH contacts and specialist reviews
Good Prescribing
up-to-date
evidence-based
cost-effective
safe
informed (patient & prescriber)
Prescribing Errors
wrong dosage
right drug prescribed to the wrong patient
inappropriate repeat prescribing
new drug interacts with existing medication
inappropriate medication
failure to monitor treatment
failure of communication between doctor and patient
Prescribing errors in hospital inpatients Postgraduate Medicine via NELM Nov 2011
Guidelines for design of in-patient prescription charts
Guidelines for design of in-patient prescription charts
Repeat prescriptions
MPS Factsheet Repeat Prescribing
Anyone signing a prescription becomes the ‘responsible prescriber’, and should be cognisant of the advice provided by the GMC in Good Medical Practice, paragraph 3(b), ie in providing care you must:
“Prescribe drugs or treatment, including repeat prescriptions, only when you have adequate knowledge of the patient’s health and are satisfied that the drugs or treatment serve the patient’s needs.”
GMC Good Practice in Prescribing Medicines – Guidance for Doctors: the paragraphs that relate to repeat prescribing are 30-31 and can be summarised as follows:
Safeguards should be put in place to ensure that:
* The patient receives the correct treatment at the correct dose.
* Each prescription is regularly reviewed so that drugs are not issued that are no longer required.
* Arrangements should be in place to ensure that any requisite monitoring is undertaken.
It is also important that there are safeguards in place to prevent a patient receiving a drug to which they are allergic, where there is an absolute contraindication or where there may be a significant interaction with other treatments, (including over-the-counter preparations).
Repeat Prescribing Protocol Bolton
Repeat prescribing enables patients to obtain further supplies of an ongoing medicine without having to see the doctor. Accounts for 65-70% of GP prescriptions and 80% of GP prescribing costs but
lends itself to mistakes, complaints and litigation so take rigorous clinical care when signing each prescription
Practices should have a written policy that is reviewed at least annually.
Familiarise yourself with the system so you can educate your patients accordingly.
Production involves receiving requests and producing a prescription. Usually delegated to a practice receptionist
Management control usually delegated to a trained staff member, comprising four elements:
1. Authorisation check
2. Compliance check
3. Review date ensuring that every patient has a clear indicator of when therapy should be removed
4. Flagging ensuring that each patient due for review is brought to the prescriber’s attention
Clinical control this is the doctor’s, or other qualified prescriber’s responsibility, and involves two tasks:
1. Authorisation the decision that a repeat prescription is appropriate, the prescriber being satisfied that the drug is effective, well tolerated and still needed
2. Periodic review a review of the patient and the medication to ensure that treatment is still effective, appropriate and well tolerated.
The prescriber makes an informed decision as to whether to continue, change or stop medication
Dealing with repeat prescription requests
Repeat prescriptions may be generated by a trained staff member but must be checked and signed by a doctor.
Check all the details on the prescription. If queries arise, access the medical record, discuss it with the partner who knows the patient well, or contact the
patient in writing or by phone or ask them to make an appointment, in order to satisfy yourself that this prescription is appropriate.
Don’t sign if you are not sure.
If a review is due you may agree to prescribe a short supply but ensure the patient comes in for a review before the next prescription is issued.
Reception staff will be responsible for sending out prescriptions or leaving them for collection, usually in an indexed box.
You may be asked by reception staff to sign ad hoc repeat prescriptions for patients that ‘can’t wait’, for example, going on holiday.
Always adhere to good prescribing practice particularly when under pressure.
Signing Repeat Prescriptions Signing repeat prescriptions (First published in the NASGP Newsletter June-July 2006)
The simple advice here is to try not to get involved in repeat prescribing. This is an area fraught with risk for locums. If you don’t know the patients or their
medical histories, this could compromise their care. The last thing you want is a batch of 100 prescriptions to sign off under the pressures of time and record-checking.
Nevertheless, you may be unable to avoid this task if your services are required long-term or in a singlehanded practice. In this case, you should agree what
will be expected of you regarding repeat prescribing in your terms with the practice in advance.
It is important to clarify:
Will you be expected to sign repeat prescriptions?
Does the practice have a protocol for safe repeat prescribing? (Some locums ask for the practice to state in writing that this system is robust and checked regularly.)
What extra time you will need and any supplementary fee for carrying out repeat prescribing work.
When using an unfamiliar electronic prescribing system:
Specific review period or dates should be entered and observed.
Don’t ignore computer warnings of overor under-use of medication.
Prescriptions should be issued with caution if a review with the patient is overdue. Make sure appropriate arrangements for timely follow up
are in place.
Add appropriate computer messages, eg ‘No more methodone until seen’, with the date and your initials.
From a patient safety and risk management perspective, the suggestions below may help.
Familiarise yourself with the practice’s repeatprescribing protocols.
Some medication is unsuitable for repeat prescribing, so a face-to-face consultation would be essential in cases such as night sedation,
antidepressants in the suicidal and NSAIDs inthe elderly.
Don’t issue a prescription for an item you feel uncomfortable with, eg hypnotics, strong analgesics or anti-depressants.
Refresh your memory on the National Prescribing Centre’s guide – Saving time, helping patients: A good practice guide to quality repeat prescribing. Repeat Dispensing & eTP Repeat dispensing
This new initiative started development nationwide from 2004. It allows for patients with stable longterm conditions to be issued a single prescription that can be dispensed at intervals by a pharmacist of their choice. As the scheme develops it should include nurse and pharmacy prescribers. The system aims to reduce the need for GP practice generated ‘repeats’ and hopefully free up some GP time, as well as making use of pharmacists’ skills andreducing waste.
Receptionist input to quality and safety in repeat prescribing BMJ Nov 2011
Repeat Prescriptions ETP
Medication review
Systematic review of the continuing need and safety of a patient’s medicine.
Effective medication reviews reduce the likelihood of medicine-related problems. The review is usually undertaken by the GP who best knows the patient, but can also be done by pharmacists, practice nurses and nurse practitioners with special training. Document the reviews in the medical record.
Is the patient taking the medications you think they are taking?
Review the original and continuing requirement for each drug and dose
Is it still appropriate?
Is it effective?
Is it the most cost-effective?
Is it being taken properly and are directions clear?
Do they understand the purpose of the drugs?
Are they happy to continue taking them?
Ask specifically about:
Side effects (consider dose or drug changes if significant)
Alcohol intake
Other non-prescribed medications also taken (OTC, herbal and homeopathic treatments) and check interactions with these where known
Institute relevant monitoring tests (including blood tests/BP)
ensure the patient understands the need for and frequency of these chase up results and notify the patient of any necessary drug changes
Clarify with the patient, with written instructions if necessary, and document current regime, including new drugs and whether they are additions or substitutes
changes made at review
tests initiated with results
arrangement for next review
The nGMS contract includes several quality markers dependent on medication reviews under both medicines management and chronic disease management
| NO TEARS tool for medication review |
|---|
| Need and indication |
| Open questions |
| Tests and monitoring |
| Evidence and guidelines |
| Adverse events |
| Risk reduction or prevention |
| Simplification and switches |
| Student BMJ, 12: 349-392 |
- Making best use of medicines: Department of Health / King’s Fund
- Patient follow-up and monitoring medicines
Blacklist limited list ACBS and SLS
Pharmaceutical Services Negotiating Committee – Prescribing Rights
ACBS Advisory Committee on Borderline Substances
There are borderline substances which can only be prescribed in certain situations, e.g. gluten-free products for those with coeliac disease or Wysoy for babies with lactose intolerance.
If not prescribed for this specific reason payment will be withheld for your error. When prescribing a borderline substance for a permitted indication write ‘ACBS’ next to the prescribed product the prescription will then normally pass through with no payment deducted
- mhra.gov.uk ACBS
- easternandcoastalkent.nhs.uk ACBS
- scotland.gov.uk non-medical prescribing borderline products
- psnc.org.uk disallowed_items
Blacklisted drugs
Items that the NHS will not supply.
Can be found in Schedule 1 to the NHS (General Medical Services Contracts) (Prescription of Drugs etc) Regulations 2004 and also in Part XVIIIA of the Drug Tariff.
If a branded product is listed on the blacklist, the NHS cannot prescribe it unless:
the prescription has been written generically and the generic product is not blacklisted
the generic name has an official title (ie it’s found at the head of a section in the reference books of the British Pharmacopoeia, British National Formulary, International Pharmacopoeia, or Dental Practitioners’ Formulary.
For example:
Laxoberal is blacklisted, but its generic name sodium picosulphate is not blacklisted, so a prescription for sodium picosulphate could be written
Regaine is blacklisted, but so is its generic name minoxidil, so it couldn’t be prescribed on the NHS.
If a blacklisted drug is prescribed by an NHS doctor, pharmacy staff will return the prescription so an alternative can be prescribed.
In some cases (such as Paramol) the drug is available over the counter so it could be sold to the patient without prescription.
Examples of blacklisted drugs include Calpol (infant paracetamol), sunscreens and vitamins.
Certain tranquillisers, cough mixtures, analgesics, antacids and laxatives are “blacklisted” by the NHS and can only be prescribed on a private prescription
eg. Frisium, Dalmane, etc. but note Frisium can be prescribed for epileptics if the FP10 is endorsed ‘S38′
Selected List Scheme SLS
may only be prescribed under certain circumstances, ie for particular patient groups and the purpose listed.
eg erectile dysfunction medication is allowed if a patient has a medical condition such as Parkinson’s, while it’s possible to get a Niferex Elixir Paediatric Dropper Bottle for premature babies to treat iron deficiency.
The doctor must mark the prescription ‘SLS’ or the pharmacy staff won’t be able to dispense it.
The selected list is in Schedule 2 to the NHS (General Medical Services Contracts) (Prescription of Drugs etc) Regulations 2004 and can be found in part XVIIIB of the Drug Tariff.
Sunscreens on FP10
Sunsense® Ultra lotion SPF 50
Delph® lotion SPF 30
Uvistat® sun cream SPF 50 – this is fragrance-free
The above sunscreens are prescribable for skin protection in photosensitivity disorders resulting from e.g. vitiligo, radiotherapy, rosacea and recurrent herpes simplex. Preparations with SPF less than 30 should not normally be prescribed. Endorse as ACBS (Advisory Council on Borderline Substance).
Medical exemptions NHS prescription charges
NHS prescriptions prepayment certificates
| Five rights and three checks of medicine administration |
|---|
| 5 Rights |
| Right Patient |
| Right Medication |
| Right Dose |
| Right Time |
| Right Route |
| (6 Right documentation) |
| 3 Checks |
| Check the label when getting from storage |
| Check the label with the drug chart |
| Recheck prior to administration |
6 rights of medication administration
Safer Prescribing over the phone
hacking-medschool/telephone consultations
Prescriptions, with the exception of controlled drugs, can be telephoned through to a local pharmacist for collection by patients.
This can be useful OOH or following a telephone consultation, but should only be undertaken if you are confident of your decision to initiate or continue treatment without the need for a face-to-face consultation.
Send the written prescription to the pharmacist promptly.
Be particularly diligent in documenting telephone directions for medication in the patient’s notes.
Prescription fraud
http://www.nhsbsa.nhs.uk/396.aspx
Circle the quantity issued, or duration of course
Rule a diagonal line though any blank space (computer will do so automatically)
Countersign/initial any alterations
Keep prescriptions out of sight (in consulting room, car, at reception)
Do not leave patients unattended in your room where they have
access to prescriptions
Keep prescriptions in a locked drawer
Take few prescriptions on visits
Handover / transfer of care (prescribing)
Drug and product liability
Drug manufacturers are liable for their products under the Consumer Protection Act 1987 and patients can claim compensation from them should they suffer injury as a result of specific drug use.
To avoid this liability falling to you as the GP, you must ensure the patient is fully informed about the appropriate use of the drug and keep adequate records documenting:
date of prescription
nature of illness
tests to establish diagnosis
quantity prescribed
warnings of side effects
problems experienced by the patient whilst taking the drug
Document clearly the dose, batch number and expiry date of any drugs or injections you administer.
Dispensing
Dispensing doctors are paid for dispensing drugs via a complicated drug tariff system.
The fee for a prescription is currently worked out as:
Basic drug price + 10.50/0 + A container allowance + A dispensing fee, which reduces the more prescriptions per month dispensed + A VAT allowance
Non-dispensing doctors may still buy in and administer certain drugs to patients (notably injections, IUCDs and sutures) and claim on the above system by submitting the relevant FP1Os to the Prescription Pricing Authority by the fifth day of the following month.
Apart from injections of emergency drugs, vaccinations (including ‘flu vaccinations) and
steroid-containing injections (e.g. DepoMedrone) are common examples of drugs that can be ‘dispensed’ by non-dispensing doctors Prescription Charges & Exemptions NHS prescriptions charges are set by government and increase most years.
OTC Meds
Proprietary Association of Great Britain OTC directory
Self Care
Prescribing Specials
NPC Prescribing Specials – Five guiding principles for prescribers
Private Prescriptions
lloydspharmacy.com PrivatePrescriptions
CDs Controlled Drugs
Marked CD in the BNF
Prescription must show:
patient’s name and address
drugform
dose, strength and the total quantity in words and figures.
your address, signature and handwritten date
Pharmacists are not legally allowed to dispense unless the prescription is completed correctly.
The use of controlled drugs is governed by the Misuse of Drugs Act 1971 and the Misuse of Drugs Regulations amended 1985.
Registered medical practitioners are allowed to ‘possess and supply CDs when acting in a professional capacity’ with the exception of schedule I drugs.
Doctors can be prosecuted for contravention of any of these regulations.
CDs must be kept in a locked compartment or receptacle and must be in a locked case if left in a locked car boot.
The practice must keep a register to record supply and use of Schedule 2 CDs which the police or Home Office inspectors have a right to inspect every 2 years.
If you keep Schedule 2 drugs in your bag you must have a separate register for this. Restocking your bag with a CD must be witnessed. Any destruction of a Schedule 2 CD must be witnessed by somebody authorised to do so, e.g. police officer, or PCO medical director.
Advise patients taking CDs and going abroad that they may require a specific licence from the Home Office, particularly if taking sizeable quantities
of Schedule 2 and 3 drugs. Doctors carrying CDs abroad when accompanying patients may also need a licence.
Schedule 2 drugs (including morphine, diamorphine and pethidine) must be kept under lock and key.
Drug misusers no longer have to be registered with the Home Office but doctors should report cases to the local Drug Misuse Database (details in the BNF)
Instalments and ‘repeats’
A prescription may order a Controlled Drug to be dispensed by instalments; the amount of instalments and the intervals to be observed must be specified
Prescriptions ordering ‘repeats’ on the same form are not permitted for Controlled Drugs in Schedules 2 or 3.
A prescription for a Controlled Drug in Schedules 2, 3, or 4 is valid for 28 days from the date stated thereon.
Department of Health guidance
Guidance (June 2006) issued by the Department of Health in England on
prescribing and dispensing of Controlled Drugs requires:
in general, prescriptions for Controlled Drugs in Schedules 2, 3, and 4
to be limited to a supply of up to 30 days’ treatment; exceptionally, to
cover a justifiable clinical need and after consideration of any risk, a
prescription can be issued for a longer period, but the reasons for the
decision should be recorded on the patient’s notes;
the patient’s identifier to be shown on NHS and private prescriptions for
Controlled Drugs in Schedules 2 and 3.
Further information is available at www.dh.gov.uk/controlleddrugs.
For a sample prescription, see above CDs Private Prescriptions Private prescriptions for Controlled Drugs in Schedules 2 and 3 must be
written on specially designated forms provided by Primary Care Trusts in
England, Health Boards in Scotland, Local Health Boards in Wales, or the
Northern Ireland Central Services Agency; in addition, prescriptions must
specify the prescriber’s identification number. Prescriptions to be supplied by
a pharmacist in hospital are exempt from the requirements for private
prescriptions.
Controlled Drugs Storing Dispensing & Record Keeping
Destruction
Patient returns may be destroyed by the Practice without an outside witness or any record but it is good practice to make a full record and have a signed witness.
Unissued, outdated stock cannot be destroyed without an approved outside witness. Witnesses may be any serving Police Officer, Home Office Inspectors, persons authorised by The Secretary of State and RPSGB inspectors, including certain Medical or Pharmaceutical advisors.
Controlled Drugs, The Law, Probity and Good Practice by Nigel Morley, £20, Surelines Pharmaceutical Services. Telephone 01604 859000
CDs Record Keeping
Records of the receipt and supply of schedule 2 CDs must be kept in a CDR.
All healthcare professionals who hold schedule 2 CD stock must keep their own CDR and are personally responsible for keeping this accurate and up to
date CDRs may be maintained either in a paper bound or electronic format.
Safer Management of Controlled Drugs (CDs): Changes to Record Keeping Requirements
From 1st February 2008, the regulations require the following information to be recorded in the CDR, under the following specified headings, when CDs are obtained:
Date supply obtained
Name and address from whom obtained (e.g. wholesaler, pharmacy)
Quantity obtained
When CDs are supplied to patients (in response to prescriptions) or to practitioners (in response to requisitions), the regulations require information
to be recorded in the CDR, under the following specified headings:
Date supplied
Name and address of person or firm supplied
Detail of authority to possess prescriber or licence holder’s details
Quantity and form in which supplied
Additional information that may be recorded
The regulations make it clear that the record keeping requirements for CDRs set out in the regulations are a minimum. They do not prevent any person
required to keep a CDR from including additional related information that will help to guarantee the integrity and accuracy of the audit trail.
The following information MAY (not must) be recorded in the CD register:
a) Running balances
b) Prescriber identification number (ie. the 6 digit private doctor code or the NHS prescriber code) and/or the professional registration number of the
prescriber where known and also the name and professional registration number of the healthcare professional supplying the CD.
Once electronic registers and electronic prescribing for CDs are in widespread use and subject to parliamentary approval, the Government will mandate the
inclusion of running balances and prescriber and supplier identification. Since CDs supplied by pharmacies can involve several pharmacists, it should be the
name of the pharmacist who makes or supervises the supply of the CD to the patient or his/her representative, whose name and professional registration number are entered in the CDR.
The Misuse of Drugs Regulations 2001 define the classes of person who are authorised to supply and possess controlled drugs while acting in their professional capacities and lay down the conditions under which these activities may be carried out.
Drugs are divided into five schedules each specifying the requirements governing activities such as import, export, production, supply, possession, prescribing, and record-keeping which apply to them.
| CD schedules and classes | |
|---|---|
| Schedule 1Cannabis LSD no therapeutic use (except research) | includes drugs such as cannabis and lysergide which are not used medicinally. Possession and supply are prohibited except in accordance with Home Office authority |
| Schedule 2Opiates and major stimulants | includes drugs such as diamorphine (heroin), morphine, remifentanil, pethidine, secobarbital, glutethimide, amfetamine, and cocaine and are subject to the full controlled drug requirements relating to prescriptions, safe custody (except for secobarbital), the need to keep registers, etc. (unless exempted in Schedule 5) |
| Schedule 3Most barbiturates and some minor stimulants | includes the barbiturates (except secobarbital, nowSchedule 2), buprenorphine, diethylpropion, mazindol, meprobamate,midazolam, pentazocine, phentermine, and temazepam. They are subject to the special prescription requirements (except for temazepam) but not to the safe custody requirements (except for buprenorphine, diethylpropion, and temazepam) nor to the need to keep registers (although there are requirements for the retention of invoices for 2 years). |
| Schedule 4Benzodiazepines and anabolic steroids | includes in Part I benzodiazepines (except temazepam and midazolam, which are in Schedule 3) and zolpidem, which are subject to minimal control. Part II includes androgenic and anabolicsteroids, clenbuterol, chorionic gonadotrophin (HCG), nonhuman chorionic gonadotrophin, somatotropin, somatrem, and somatropin.Controlled drug prescription requirements do not apply and Schedule 4 Controlled Drugs are not subject to safe custody requirements |
| Schedule 5CDs combined with other drugs with minimal risk of abuse | includes those preparations which, because of their strength, are exempt from virtually all Controlled Drug requirements other than retention of invoices for two years. |
The penalties applicable to offences involving the different drugs are graded broadly according to the harmfulness attributable to a drug when it is misused and for this purpose the drugs are defined in the following three classes
| CD Classes | |
|---|---|
| Class A | alfentanil, cocaine, diamorphine (heroin), dipipanone, lysergide (LSD), methadone, methylenedioxymethamfetamine (MDMA, ‘ecstasy’), morphine, opium, pethidine, phencyclidine, remifentanil, and class B substances when prepared for injection |
| Class B | oral amphetamines, barbiturates, cannabis, codeine, ethylmorphine, glutethimide, pentazocine, phenmetrazine, and pholcodine |
| Class C | certain drugs related to the amphetamines such as benzfetamine and chlorphentermine, buprenorphine, diethylpropion, mazindol, meprobamate, pemoline, pipradrol, most benzodiazepines, zolpidem, androgenic and anabolic steroids, clenbuterol, chorionic gonadotrophin (HCG), non-human chorionic gonadotrophin, somatotropin, somatrem, and somatropin |
CDs taking CDs abroad
http://hacking-medschool.com/taking-controlled-drugs-abroad-2
Doping control in sport
Indication prescribing
Prescribing incentive schemes and prescribing indicators
brighton and hove pct prescribing incentive scheme
PACT
PACT – Prescribing Analysis and Cost Data
Scotland Scottish Prescribing Analysis SPA
Northern Ireland Prescribing Prices Information NIPPI
PACT data are issued every 3 months to all GP principals by the Prescription Pricing Authority (PPA), which processes all dispensed NHS prescriptions.
It provides summaries of GPs’ individual and practice prescribing with information on the number of individual items and costs of the prescriptions dispensed (not issued).
Local and national comparisons are provided together with data for the previous year.
Drugs are divided into the main six BNF therapeutic groups and the 20 leading prescriptions in the practice are identified.
Prescribing advisors
Pharmacists
Medicines Management
Regional Medicines Information Service
Indicative prescribing budgets
From 1st April 1991 GPs will be subject to the ‘indicative prescribing scheme’. Note, however, that fund holders will have prescribing costs
included with their general fund. The indicative prescribing scheme willwork as follows:
1. The FHSA will allocate the practice an amount to cover its prescribing costs. The FHSA will take into account the current prescribing pattern of the practice, the average prescribing costs in the area and any special factors relevant to the practice (e.g. a few patients on very expensive medication)
2. Every month the practice will receive, from the Prescription Pricing Authority, a statement of the amount spent by the practice on prescribing and this will be a way of monitoring how your expenditure is matching the allocation of funds
3. At the end of the Financial Year the FHSA will examine the practice’s prescribing expenditure. If the practice has exceeded the allocated amount there are several possibilities viz:
(i) After explanation from the practice, the FHSA may accept that the increased expenditure was justified on the basis of patient need
(ii) If the FHSA considers the prescribing to be excessive without clinical justification, it can refer the GP(s) to a hearing in front of a committee of three doctors and this may result in the withholding of income from those GPs
4. Each FHSA will appoint a medical adviser to liaise with practices where prescribing is deemed to be a problem (this includes underprescribing as well as over-prescribing)
5. Practice formularies are to be encouraged but not compulsor PACT Prescribing Analysis and Cost data Also known as SPA: Scottish Prescribing Analysis, or NIPPI: Northern Ireland Prescribing Prices Information.
Community pharmacists
Community Pharmacy Services – briefing for GP practices Aug 2011
Prescribing off license
Drugs sold in the UK must have a Product Licence (marketing authorisation) granted by the Medicines and Healthcare Products Regulatory Agency, which effectively confirms its effectiveness and safety and that it gives overall benefit.
There may be occasions when unlicensed drugs can be prescribed, or licensed drugs prescribed for indications outside their licence, e.g. for use in children.
In such cases the patient must be fully informed that the drug is unlicensed and that all actions and side effects may not be known; document this in their notes.
The drug may be available direct from the manufacturer on a ‘named patient’ only basis.
This is not that common in general practice, andnso you may need to seek advice from your colleagues and pco pharmacy advisor.
Compliance aids
Generic prescribing
by drug name rather than proprietary (brand) name is cost-effective, encourages consistency and is used as a measure of good prescribing.
When presented with a generic prescription, pharmacists are not obliged to dispense a particular brand.
You should explain to the patient that, on occasion, they may get a different looking drug or even a different name on the package but this should not affect their treatment.
Non Generics / Exceptions
We are encouraged to prescribe generically (ie. frusemide rather than lasix) but there are also arguments against (bioavailability, presentation, etc.)
There are a few exceptions where it is medically necessary to prescribe a particular brand:
Drugs with a narrow therapeutic index
Lithium
Cyclosporin
Modified release preparations (where different formulations give different bioavailability)
Theophylline/aminophylline
Diltiazem/nifedipine
Anticonvulsants
Branded Generics Pharma Times Aug 2011
Generic drug names ready reference
| Generic drug names ready reference | ||
|---|---|---|
| enalapril | innovaxe | innozide |
| lisinopril | zestril | carace plus |
| perindopril | coversyl | coversyl plus |
| quinapril | accupro | accureyic |
| ramipril | tritace | |
| candesartan | amias | |
| irbesartan | approval | |
| losartan | cozaar | |
| valsartan | diovan | |
ScriptSwitch
http://hacking-medschool.com/scriptswitch
useful tool for cost effective/rational prescribing (as dictated by local MMCs) but the prompts can introduce unnecessary potential for prescribing error
Prescribing over the phone
hacking-medschool/prescribing over the phone/
Concordanc and Compliance
- NPC PDAs
- Compliance becomes concordance BMJ Mar 1997
- Concordance Monica Arora Bath VTS 2006 ppt
- Consultation Skills Dr. Ashraf Ahmed
Dressings and appliances
hacking-medschool/wound-care-dressings-products
Compression stockings
| Compression Stockings Classes | ||
|---|---|---|
| Class1 | mild ankle swelling | least compressive |
| Class2 | recurrent venous ulcer formation | |
| Class3 | lymphoedema or severe post-thrombotic venous insufficiency | most compressive |
All classes come in above and below-knee varieties.
Doppler studies are needed before prescribing Grade 2 or 3 in patients with PVD
GP Clinical Survival Guide
Tubigrip sizes
| Tubigrip Sizes | |
|---|---|
| A | Infant feet and arms |
| B | Small hands and arms |
| C | Medium arms, small ankles |
| D | Large arms, medium ankles, small knees |
| E | Large ankles, medium knees, small thighs |
| F | Large knees, medium thighs |
| G | Large thighs. |
Appliances



