Learning Objectives
By the end of this section, you will be able to:
- Identify the three blood variables considered when making a diagnosis of acidosis or alkalosis
- Identify the source of compensation for blood pH problems of a respiratory origin
- Identify the source of compensation for blood pH problems of a metabolic/renal origin
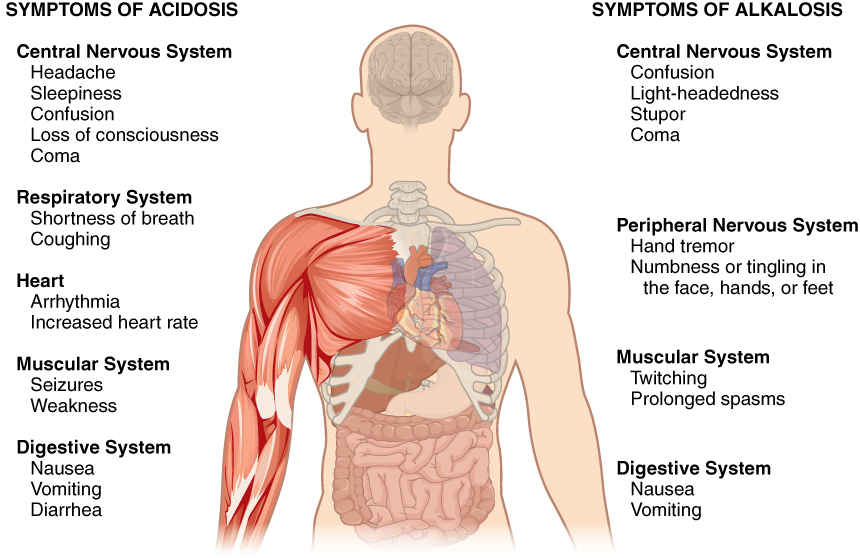
Normal arterial blood pH is restricted to a very narrow range of 7.35 to 7.45. A person who has a blood pH below 7.35 is considered to be in acidosis (actually, “physiological acidosis,” because blood is not truly acidic until its pH drops below 7), and a continuous blood pH below 7.0 can be fatal. Acidosis has several symptoms, including headache and confusion, and the individual can become lethargic and easily fatigued (Figure 14.15.1). A person who has a blood pH above 7.45 is considered to be in alkalosis, and a pH above 7.8 is fatal. Some symptoms of alkalosis include cognitive impairment (which can progress to unconsciousness), tingling or numbness in the extremities, muscle twitching and spasm, and nausea and vomiting. Both acidosis and alkalosis can be caused by either metabolic or respiratory disorders.
As discussed earlier in this chapter, the concentration of carbonic acid in the blood is dependent on the level of CO2 in the body and the amount of CO2 gas exhaled through the lungs. Thus, the respiratory contribution to acid-base balance is usually discussed in terms of CO2 (rather than of carbonic acid). Remember that a molecule of carbonic acid is lost for every molecule of CO2 exhaled, and a molecule of carbonic acid is formed for every molecule of CO2 retained.
Metabolic Acidosis: Primary Bicarbonate Deficiency
Metabolic acidosis occurs when the blood is too acidic (pH below 7.35) due to too little bicarbonate, a condition called primary bicarbonate deficiency. At the normal pH of 7.40, the ratio of bicarbonate to carbonic acid buffer is 20:1. If a person’s blood pH drops below 7.35, then he or she is in metabolic acidosis. The most common cause of metabolic acidosis is the presence of organic acids or excessive ketones in the blood. Table 14.15.1 lists some other causes of metabolic acidosis.
Table 14.15.1. Common causes of metabolic acidosis and blood metabolites
| Cause | Metabolite |
| Diarrhoea | Bicarbonate |
| Uraemia | Phosphoric, sulfuric, and lactic acids |
| Diabetic ketoacidosis | Increased ketones |
| Strenuous exercise | Lactic acid |
| Methanol | Formic acid* |
| Paraldehyde | β-Hydroxybutyric acid* |
| Isopropanol | Propionic acid* |
| Ethylene glycol | Glycolic acid, and some oxalic acids and formic acids* |
| Salicylate/aspirin | Sulfosalicylic acid (SSA)* |
| * Acid metabolites from ingested chemical |
The first three of the eight causes of metabolic acidosis listed are medical (or unusual physiological) conditions. Strenuous exercise can cause temporary metabolic acidosis due to the production of lactic acid. The last five causes result from the ingestion of specific substances. The active form of aspirin is its metabolite, sulfasalicylic acid. An overdose of aspirin causes acidosis due to the acidity of this metabolite. Metabolic acidosis can also result from uraemia, which is the retention of urea and uric acid. Metabolic acidosis can also arise from diabetic ketoacidosis, wherein an excess of ketones is present in the blood. Other causes of metabolic acidosis are a decrease in the excretion of hydrogen ions, which inhibits the conservation of bicarbonate ions, and excessive loss of bicarbonate ions through the gastrointestinal tract due to diarrhoea.
Metabolic Alkalosis: Primary Bicarbonate Excess
Metabolic alkalosis is the opposite of metabolic acidosis. It occurs when the blood is too alkaline (pH above 7.45) due to too much bicarbonate (called primary bicarbonate excess).
A transient excess of bicarbonate in the blood can follow ingestion of excessive amounts of bicarbonate, citrate, or antacids for conditions such as stomach acid reflux—known as heartburn. Cushing’s disease, which is the chronic hypersecretion of adrenocorticotrophic hormone (ACTH) by the anterior pituitary gland, can cause chronic metabolic alkalosis. The oversecretion of ACTH results in elevated aldosterone levels and an increased loss of potassium by urinary excretion. Other causes of metabolic alkalosis include the loss of hydrochloric acid from the stomach through vomiting, potassium depletion due to the use of diuretics for hypertension, and the excessive use of laxatives.
Respiratory Acidosis: Primary Carbonic Acid/CO2 Excess
Respiratory acidosis occurs when the blood is overly acidic due to an excess of carbonic acid, resulting from too much CO2 in the blood. Respiratory acidosis can result from anything that interferes with respiration, such as pneumonia, emphysema, or congestive heart failure.
Respiratory Alkalosis: Primary Carbonic Acid/CO2 DEFICIENCY
Respiratory alkalosis occurs when the blood is overly alkaline due to a deficiency in carbonic acid and CO2 levels in the blood. This condition usually occurs when too much CO2 is exhaled from the lungs, as occurs in hyperventilation, which is breathing that is deeper or more frequent than normal. An elevated respiratory rate leading to hyperventilation can be due to extreme emotional upset or fear, fever, infections, hypoxia, or abnormally high levels of catecholamines, such as adrenaline and noradrenaline. Surprisingly, aspirin overdose—salicylate toxicity—can result in respiratory alkalosis as the body tries to compensate for initial acidosis.
Compensation Mechanisms
Various compensatory mechanisms exist to maintain blood pH within a narrow range, including buffers, respiration and renal mechanisms. Although compensatory mechanisms usually work very well, when one of these mechanisms is not working properly (like kidney failure or respiratory disease), they have their limits. If the pH and bicarbonate to carbonic acid ratio are changed too drastically, the body may not be able to compensate. Moreover, extreme changes in pH can denature proteins. Extensive damage to proteins in this way can result in disruption of normal metabolic processes, serious tissue damage and ultimately, death.
Respiratory Compensation
Respiratory compensation for metabolic acidosis increases the respiratory rate to drive off CO2 and readjust the bicarbonate to carbonic acid ratio to the 20:1 level. This adjustment can occur within minutes. Respiratory compensation for metabolic alkalosis is not as adept as its compensation for acidosis. The normal response of the respiratory system to elevated pH is to increase the amount of CO2 in the blood by decreasing the respiratory rate to conserve CO2. There is a limit to the decrease in respiration, however, that the body can tolerate. Hence, the respiratory route is less efficient at compensating for metabolic alkalosis than for acidosis.
Metabolic Compensation
Metabolic and renal compensation for respiratory diseases that can create acidosis revolves around the conservation of bicarbonate ions. In cases of respiratory acidosis, the kidney increases the conservation of bicarbonate and secretion of H+ through the exchange mechanism discussed earlier. These processes increase the concentration of bicarbonate in the blood, re-establishing the proper relative concentrations of bicarbonate and carbonic acid. In cases of respiratory alkalosis, the kidneys decrease the production of bicarbonate and reabsorb H+ from the tubular fluid. These processes can be limited by the exchange of potassium by the renal cells, which use a K+-H+ exchange mechanism (antiporter).
Diagnosing Acidosis and Alkalosis
Lab tests for pH, CO2 partial pressure (pCO2) and HCO3– can identify acidosis and alkalosis, indicating whether the imbalance is respiratory or metabolic, and the extent to which compensatory mechanisms are working. The blood pH value, as shown in Table 14.15.2, indicates whether the blood is in acidosis, the normal range, or alkalosis. The pCO2 and total HCO3– values aid in determining whether the condition is metabolic or respiratory, and whether the patient has been able to compensate for the problem. Table 14.15.2 lists the conditions and laboratory results that can be used to classify these conditions. Metabolic acid-base imbalances typically result from kidney disease and the respiratory system usually responds to compensate.
Table 14.15.2. Types of acidosis and alkalosis
| pH | pCO2 | Total HCO3– | |
| Metabolic acidosis | Decreases | Normal, then decreases | Decreases |
| Respiratory acidosis | Decreases | Increases | Normal, then increases |
| Metabolic alkalosis | Increases | Normal, then increases | Increases |
| Respiratory alkalosis | Increases | Decreases | Normal, then decreases |
Reference values (arterial): pH: 7.35-7.45; pCO2: male: 35-48 mmHg, female: 32-45 mmHg; total venous bicarbonate: 22-29 mM.
Metabolic acidosis is problematic, as lower-than-normal amounts of bicarbonate are present in the blood. The pCO2 would be normal at first, but if compensation has occurred, it would decrease as the body re-establishes the proper ratio of bicarbonate and carbonic acid/CO2.
Respiratory acidosis is problematic, as excess CO2 is present in the blood. Bicarbonate levels would be normal at first, but if compensation has occurred, they would increase in an attempt to re-establish the proper ratio of bicarbonate and carbonic acid/CO2.
Alkalosis is characterised by a higher-than-normal pH. Metabolic alkalosis is problematic, as elevated pH and excess bicarbonate are present. The pCO2 would again be normal at first, but if compensation has occurred, it would increase as the body attempts to re-establish the proper ratios of bicarbonate and carbonic acid/CO2.
Respiratory alkalosis is problematic, as CO2 deficiency is present in the bloodstream. The bicarbonate concentration would be normal at first. When renal compensation occurs, however, the bicarbonate concentration in blood decreases as the kidneys attempt to re-establish the proper ratios of bicarbonate and carbonic acid/CO2 by eliminating more bicarbonate to bring the pH into the physiological range.
Section Review
Acidosis and alkalosis describe conditions in which a person’s blood is, respectively, too acidic (pH below 7.35) and too alkaline (pH above 7.45). Each of these conditions can be caused either by metabolic problems related to bicarbonate levels or by respiratory problems related to carbonic acid and CO2 levels. Several compensatory mechanisms allow the body to maintain a normal pH.
