Learning Objectives
By the end of this section, you will be able to:
- Explain the mechanism of action of diuretics
- Explain why the differential permeability or impermeability of specific sections of the nephron tubules is necessary for urine formation
The major hormones influencing total body water are ADH, aldosterone and ANP. Circumstances that lead to fluid depletion in the body include blood loss and dehydration. Homeostasis requires that volume and osmolarity be preserved. Blood volume is important in maintaining sufficient blood pressure, and there are non-renal mechanisms involved in its preservation, including vasoconstriction, which can act within seconds of a decrease in pressure. Thirst mechanisms are also activated to promote the consumption of water lost through respiration, evaporation or urination. Hormonal mechanisms are activated to recover volume while maintaining a normal osmotic environment. These mechanisms act principally on the kidney.
Volume-Sensing Mechanisms
The body cannot directly measure blood volume, but blood pressure can be measured. Blood pressure often reflects blood volume and is measured by baroreceptors in the aorta and carotid sinuses. When blood pressure increases, baroreceptors send more frequent action potentials to the central nervous system, leading to widespread vasodilation. Included in this vasodilation are the afferent arterioles supplying the glomerulus, resulting in increased GFR and water loss by the kidneys. If pressure decreases, fewer action potentials travel to the central nervous system, resulting in more sympathetic stimulation-producing vasoconstriction, which will result in decreased filtration and GFR and water loss.
Decreased blood pressure is also sensed by the granular cells in the afferent arteriole of the JGA. In response, the enzyme renin is released. You saw earlier in the chapter that renin activity leads to an almost immediate rise in blood pressure as activated angiotensin II produces vasoconstriction. The rise in pressure is sustained by the aldosterone effects initiated by angiotensin II; this includes an increase in Na+ retention and water volume. As an aside, late in the menstrual cycle, progesterone has a modest influence on water retention. Due to its structural similarity to aldosterone, progesterone binds to the aldosterone receptor in the collecting duct of the kidney, causing the same, albeit weaker, effect on Na+ and water retention.
Cardiomyocytes of the atria also respond to greater stretch (as blood pressure rises) by secreting ANP. ANP opposes the action of aldosterone by inhibiting the recovery of Na+ by the DCT and collecting ducts. More Na+ is lost, and as water follows salt, total blood volume and pressure decline. In low-pressure states, ANP does not seem to have much effect.
ADH is also called vasopressin. Early researchers found that in cases of unusually high secretion of ADH, the hormone caused vasoconstriction (vasopressor activity, hence the name). Only later were its antidiuretic properties identified. Synthetic ADH is still used occasionally to stem life-threatening oesophagus bleeding in alcoholics.
When blood volume drops 5–10 percent, causing a decrease in blood pressure, there is a rapid and significant increase in ADH release from the posterior pituitary. Immediate vasoconstriction to increase blood pressure is the result. ADH also causes activation of aquaporin channels in the collecting ducts to affect the recovery of water to help restore vascular volume.
Diuretics and Fluid Volume
A diuretic is a compound that increases urine volume. Three familiar drinks contain diuretic compounds: coffee, tea, and alcohol. The caffeine in coffee and tea works by promoting vasodilation in the nephron, which increases GFR. Alcohol increases GFR by inhibiting ADH release from the posterior pituitary, resulting in less water recovery by the collecting duct. In cases of high blood pressure, diuretics may be prescribed to reduce blood volume and, thereby, reduce blood pressure. The most frequently prescribed anti-hypertensive diuretic is hydrochlorothiazide. It inhibits the Na+/ Cl– symporter in the DCT and collecting duct. The result is a loss of Na+ with water following passively by osmosis.
Osmotic diuretics promote water loss by osmosis. An example is the indigestible sugar mannitol, which is most often administered to reduce brain swelling after head injury. However, it is not the only sugar that can produce a diuretic effect. In cases of poorly controlled diabetes mellitus, glucose levels exceed the capacity of the tubular glucose symporters, resulting in glucose in the urine. The unrecovered glucose becomes a powerful osmotic diuretic. Classically, in the days before glucose could be detected in the blood and urine, clinicians identified diabetes mellitus by the three Ps: polyuria (diuresis), polydipsia (increased thirst) and polyphagia (increased hunger).
Regulation of Extracellular Sodium Ions (Na+)
Sodium has a strong osmotic effect and attracts water. It plays a larger role in the osmolarity of the plasma than any other circulating component of the blood. If there is too much Na+ present, either due to poor control or excess dietary consumption, a series of metabolic problems ensue. There is an increase in total volume of water, which leads to hypertension (high blood pressure). Over a long period, this increases the risk of serious complications such as heart attacks, strokes and aneurysms. It can also contribute to system-wide oedema (swelling).
Mechanisms for regulating Na+ concentration include the renin–angiotensin–aldosterone system and ADH. Aldosterone stimulates the uptake of Na+ on the apical cell membrane of cells in the DCT and collecting ducts, whereas ADH helps to regulate Na+ concentration indirectly by regulating the reabsorption of water.
Regulation of Extracellular Potassium Ions (K+)
Potassium is present in a 30-fold greater concentration inside the cell than outside the cell. A generalisation can be made that K+ and Na+ concentrations will move in opposite directions. When more Na+ is reabsorbed, more K+ is secreted; when less Na+ is reabsorbed (leading to excretion by the kidney), more K+ is retained. When aldosterone causes a recovery of Na+ in the nephron, a negative electrical gradient is created that promotes the secretion of K+ and Cl– into the lumen.
Regulation of Chloride Ions (Cl–)
Chloride is important in acid–base balance in the extracellular space and has other functions, such as in the stomach, where it combines with hydrogen ions in the stomach lumen to form hydrochloric acid, aiding digestion. Its close association with Na+ in the extracellular environment makes it the dominant anion of this compartment and its regulation closely mirrors that of Na+.
Regulation of Ca2+ and Phosphate
The parathyroid glands monitor and respond to circulating levels of Ca2+ in the blood. When levels drop too low, PTH is released to stimulate the DCT to reabsorb Ca2+ from the forming urine. When levels are adequate or high, less PTH is released and more Ca2+ remains in the forming urine to be lost. Phosphate levels move in the opposite direction. When Ca2+ levels are low, PTH inhibits reabsorption of HPO42− so that its blood level drops, allowing Ca2+ levels to rise. PTH also stimulates the renal conversion of calcidiol into calcitriol, the active form of vitamin D. Calcitriol then stimulates the intestines to absorb more Ca2+ from the diet.
Regulation of H+, Bicarbonate and pH
The acid–base homeostasis of the body is a function of chemical buffers and physiologic buffering provided by the lungs and kidneys. Buffers, especially proteins, HCO32−, and ammonia have a very large capacity to absorb or release H+ as needed to resist a change in pH. They can act within fractions of a second. The lungs can rid the body of excess acid very rapidly (seconds to minutes) through the conversion of HCO3– into CO2, which is then exhaled. It is rapid but has limited capacity in the face of a significant acid challenge. The kidneys can rid the body of both acid and base. The renal capacity is large but slow (minutes to hours). The cells of the PCT actively secrete H+ into the forming urine as Na+ is reabsorbed. The body rids itself of excess H+ and raises blood pH. In the collecting ducts, the apical surfaces of intercalated cells have proton pumps that actively secrete H+ into the luminal, forming urine to remove it from the body.
As hydrogen ions are pumped into the forming urine, it is buffered by bicarbonate (HCO3–), H2PO4– (dihydrogen phosphate ion) or ammonia (forming NH4+, ammonium ion). Urine pH typically varies in a normal range from 4.5 to 8.0.
Regulation of Nitrogen Wastes
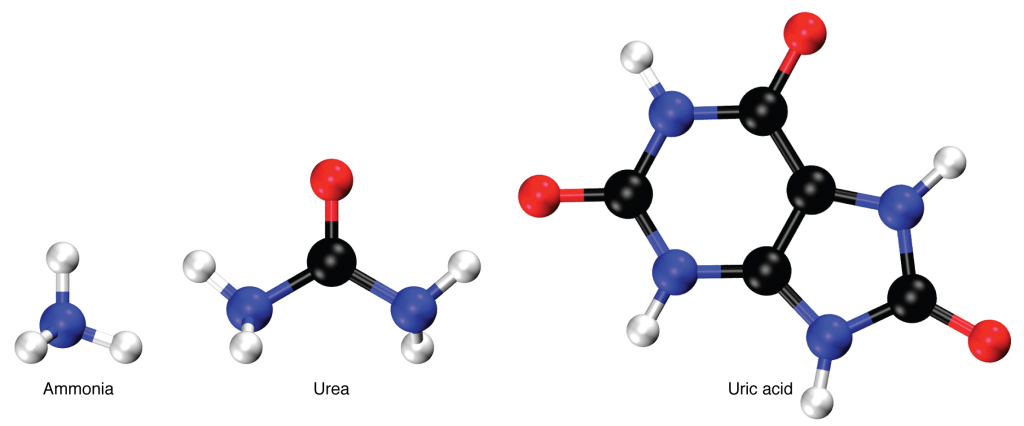
Nitrogen wastes are produced by the breakdown of proteins during normal metabolism. Proteins are broken down into amino acids, which in turn are deaminated by having their nitrogen groups removed. Deamination converts the amino (NH2) groups into ammonia (NH3), ammonium ion (NH4+), urea or uric acid (Figure 14.9.1). Ammonia is extremely toxic, so most of it is very rapidly converted into urea in the liver. Human urinary wastes typically contain primarily urea with small amounts of ammonium and very little uric acid.
Elimination of Drugs and Hormones
Water-soluble drugs may be excreted in the urine and are influenced by one or all of the following processes: glomerular filtration, tubular secretion or tubular reabsorption. Drugs that are structurally small can be filtered by the glomerulus with the filtrate. Large drug molecules such as heparin or those that are bound to plasma proteins cannot be filtered and are not readily eliminated. Some drugs can be eliminated by carrier proteins that enable secretion of the drug into the tubule lumen. There are specific carriers that eliminate basic (such as dopamine or histamine) or acidic drugs (such as penicillin or indomethacin). As is the case with other substances, drugs may be both filtered and reabsorbed passively along a concentration gradient.
Section Review
The major hormones regulating body fluids are ADH, aldosterone and ANP. Progesterone is similar in structure to aldosterone and can bind to and weakly stimulate aldosterone receptors, providing a similar but diminished response. Blood pressure reflects blood volume and is monitored by baroreceptors in the aortic arch and carotid sinuses. When blood pressure increases, more action potentials are sent to the central nervous system, resulting in greater vasodilation, greater GFR, and more water lost in the urine. ANP is released by the cardiomyocytes when blood pressure increases, causing Na+ and water loss. ADH at high levels causes vasoconstriction in addition to its action on the collecting ducts to recover more water. Diuretics increase urine volume. Mechanisms for controlling Na+ concentration in the blood include the renin–angiotensin–aldosterone system and ADH. When Na+ is retained, K+ is excreted; when Na+ is lost, K+ is retained. When circulating Ca2+ decreases, PTH stimulates the reabsorption of Ca2+ and inhibits reabsorption of HPO42−. pH is regulated through buffers, expiration of CO2 and excretion of acid or base by the kidneys. The breakdown of amino acids produces ammonia. Most ammonia is converted into less-toxic urea in the liver and excreted in the urine. Regulation of drugs is by glomerular filtration, tubular secretion and tubular reabsorption.
Review Questions
- What is GFR?
- What causes release of ANP and what effect does ANP have on GFR?
- When the RAAS is activated, what changes would you expect in GFR?
