Chapter 14: Minerals
“God sleeps in the minerals, awakens in plants, walks in animals, and thinks in man.”
-Arthur Young (1741-1820), English writer on agriculture, politics, and economics
The second category of micronutrients are minerals. Similarly to vitamins, minerals are essential to human health, can be obtained in our diet from different types of food, and are needed in very small amounts relative to the macronutrients. Unlike vitamins, minerals are inorganic meaning they do not contain carbon. Minerals are abundant in our everyday lives. From the soil in your front yard to the jewelry you wear on your body, we interact with minerals constantly. In this chapter we will discuss the 20 essential minerals that are required by the human body to remain healthy.
Learning Objectives
- Describe the functional roles, intake recommendations, dietary sources, and health consequences of excessive or inadequate intake of the seven major minerals.
- Describe the functional roles, intake recommendations, dietary sources, and health consequences of excessive or inadequate intake of the trace minerals.
14.1 Basic Concepts of Minerals
Minerals are inorganic substances in their simplest form that are vital to health and abundant in our everyday lives. From the soil in your front yard to the jewelry you wear, we interact with minerals constantly. Unlike vitamins which are classified based on their solubility, minerals are categorized by the body’s daily requirements. The amounts of each mineral found in our bodies vary greatly and therefore, so does consumption of those minerals. When there is a deficiency of an essential mineral, health problems may arise.
Minerals are not as efficiently absorbed as most vitamins and so the bioavailability of minerals can be very low. In general, minerals are better absorbed from animal-based foods. Plant-based foods often contain compounds, such as oxalate and phytate, that bind to minerals and inhibit their absorption. In most cases, if dietary intake of a particular mineral is increased, absorption will decrease. Some minerals influence the absorption of others. For instance, excess zinc in the diet can impair iron and copper absorption. Conversely, certain vitamins enhance mineral absorption. For example, vitamin C boosts iron absorption, and vitamin D boosts calcium and magnesium absorption. As is the case with vitamins, certain gastrointestinal disorders and diseases, such as Crohn’s disease and kidney disease, as well as the aging process impair mineral absorption, putting people with malabsorption conditions and the elderly at higher risk for mineral deficiencies. For more information about nutrient bioavailability, refer to Chapter 13.
14.2 Major Minerals
Major minerals are classified as minerals that are required in the diet each day in amounts larger than 100 mg. There are seven major minerals: calcium, chloride, magnesium, phosphorus, potassium, sodium, and sulfur. Many of these minerals are classified as electrolytes which can separate into charged ions when dissolved.
Sodium
Sodium is an example of an electrolyte. It is vital not only for maintaining fluid balance but also for many other essential functions. In contrast to many other minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. In fact, very little sodium is required in the diet (about 200 mg) because the kidneys actively reabsorb sodium. Kidney reabsorption of sodium is hormonally controlled, allowing for a relatively constant sodium concentration in the blood.
Another notable function of sodium is in nerve impulse transmission. Nerve impulse transmission results from the transport of sodium cations (positively charged ions) into a nerve cell, which creates a charge difference (or voltage) between the nerve cell and its extracellular environment. Similar to how a current moves along a wire, a sodium current moves along a nerve cell. Stimulating a muscle contraction also involves the movement of sodium ions as well as other ion movements.
Sodium is essential for nutrient absorption in the small intestine and also for nutrient reabsorption in the kidney. Amino acids, glucose, and water must make their way from the small intestine to the blood. To do so, they pass through cells in intestinal walls to get to the capillaries in the villi and into the blood. The transport of nutrients through intestinal cells is facilitated by the sodium-potassium pump, which involves moving sodium out of the cell, and potassium into the cell which requires ATP. This action is essential for creating conditions necessary for movement of nutrients, firing action potentials (for nerve or muscle activation), fluid balance, and more.
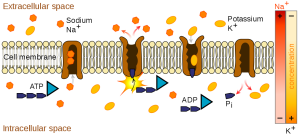
While a bit of sodium occurs naturally in some foods, more than 70% of the sodium we consume comes from processed, prepackaged, and restaurant foods. Many people are surprised by the amount of sodium in foods that do not taste particularly salty. In fact, these foods, known as the salty six are some of the top sodium sources in the US diet1:
- Breads and rolls
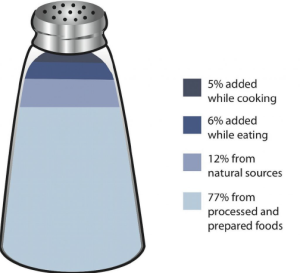
Figure 14.2.2 Salt Sources in the Diet - Pizza
- Sandwiches
- Cold cuts and cured meats
- Soup
- Burritos and tacos
As stated previously, sodium is imperative for optimal functioning in the body, however, most Americans consume more than necessary. The National Academy of Medicine (NAM) estimates that greater than 95% of men and 75% of women in America consume salt in excess of the Tolerable Upper Limit (UL). The Adequate Intake (AI) for sodium in adults aged 19-50 years old is 1,500 mg/day. Table salt is approximately 40% sodium, and 60% chloride. As a reference point, only ⅔ teaspoon of salt is needed to meet the AI for sodium.

The AI takes into account the amount of sodium lost in sweat during recommended physical activity levels and additionally provides for the sufficient intake of other nutrients, such as chloride. The UL for sodium is 2,300 mg/day for adults. Again, as a point of reference, just over one teaspoon of salt contains the 2,300 mg of sodium recommended. The UL is considered appropriate for healthy individuals but not those with hypertension (high blood pressure). Many scientific studies demonstrate that reducing salt intake reduces the risk of developing hypertension, is helpful in reducing blood pressure after hypertension is diagnosed, and reduces the risk for additional cardiovascular diseases.2
The Nutrition Facts Label (NFL) displays the amount of sodium (in mg) per serving of the food. It also provides the % Daily Value (%DV). Recall from Chapter 2 that a %DV of 5% or below means that a serving of the product is low in sodium, if a %DV is 20% or higher, that product is high in sodium and perhaps should be avoided. Food additives are often high in sodium, for example, monosodium glutamate (MSG) contains 12%DV of sodium. Additionally, baking soda, baking powder, disodium phosphate, sodium alginate, and sodium nitrate or nitrite contain a significant proportion of sodium as well. When you see a food’s NFL, you can check the ingredients list to identify the source of the added sodium. Various claims about the sodium content in foods must be in accordance with Food and Drug Administration (FDA) regulations (see Table 14.2.1).3
Table 14.2.1 Food Packaging Claims Regarding Sodium.3
| Claim | Definition |
| Light or Low in sodium | Sodium reduced by 50% from reference product |
| No salt added/Unsalted | No salt added during preparation or processing (Must also declare on package “This is not a sodium-free food” if food is not sodium-free) |
| Lightly salted | 50% less sodium than that added to similar food |
| Sodium free/Salt free | Contains less than 5 mg of sodium per serving |
| Very low salt | Contains less than 35 mg of sodium per serving |
| Low salt | Contains less than 140 mg of sodium per serving |
To decrease your sodium intake, become a salt-savvy shopper by reading the labels and ingredient lists of processed foods and choosing those lower in salt. Even better, stay away from processed foods and control the seasoning of your foods. Eating a diet with fewer salty foods diminishes salt cravings so you may need to try a lower sodium diet for a week or two before you will be satisfied with the less salty food. You can also use salt alternatives to flavor your food instead of using salt. Fresh or dried herbs like rosemary, thyme, dill, or parsley can add flavor to dishes, garlic and curry have been used for thousands of years as flavoring, and spices like cinnamon and paprika can pack a powerful flavor punch without adding sodium.4
Salt Substitutes
For those with hypertension or those looking for a way to decrease salt use, using a salt substitute for food preparation is one option. However, many salt substitutes still contain sodium, just in lesser amounts than table salt. Also, remember that most salt in the diet is not from table salt use, but from processed foods. Salt substitutes often replace the sodium with potassium. People with kidney disorders can have problems getting rid of excess potassium and are advised to avoid salt substitutes containing potassium. People with liver disorders should also avoid salt substitutes containing potassium because their treatment is often accompanied by potassium dysregulation. If this is an issue for you, check labels of salt substitutes carefully.
Too much sodium is certainly problematic, but so is too little sodium. Sweating is a homeostatic mechanism for maintaining body temperature, which influences fluid and electrolyte balance. Sweat is mostly water but also contains some electrolytes, mostly sodium and chloride. Under normal environmental conditions (i.e., not hot, humid days) water and sodium loss through sweat is negligible, but is highly variable among individuals. It is estimated that 60 minutes of high-intensity physical activity, like playing a game of tennis, can produce approximately one liter of sweat; however the amount of sweat produced is highly dependent on environmental conditions. A liter of sweat typically contains between 1-2 g of sodium and therefore exercising for multiple hours can result in a high amount of sodium loss in some people. Additionally, hard labor, especially outdoors, can produce substantial sodium loss through sweat. In either case, the lost sodium is easily replaced in the next snack or meal.
In athletes, hyponatremia, or a low blood sodium level, is not so much the result of excessive sodium loss in sweat, but rather drinking too much water. The excess water dilutes the sodium concentration in blood. Illnesses causing vomiting, sweating, and diarrhea may also cause hyponatremia. The symptoms of hyponatremia, also called water intoxication (since it is often the root cause), include nausea, muscle cramps, confusion, dizziness, and in severe cases, coma and death.
Chloride
Chloride is the primary anion (negatively charged ion) in extracellular fluid. In addition to passively following sodium, chloride has its own protein channels that reside in cell membranes. These protein channels are especially abundant in the gastrointestinal tract, pancreas, and lungs.
Chloride aids in fluid balance mainly because it follows sodium in order to maintain charge neutrality. Chloride channels also play a role in regulating fluid secretion, such as pancreatic juice into the small intestine and the flow of water into mucus.
Chloride has several other functions in the body, most importantly in acid-base balance. Blood pH is maintained in a narrow range and the number of positively charged substances is equal to the number of negatively charged substances. Proteins, such as albumin, as well as bicarbonate ions and chloride ions, are negatively charged and aid in maintaining blood pH. Hydrochloric acid (composed of chlorine and hydrogen) aids in digestion and also prevents the growth of unwanted microbes in the stomach. Immune system cells require chloride, and red blood cells use chloride anions to remove carbon dioxide from the body.
Most chloride in the diet comes from salt, which as previously stated is 60% chloride. A teaspoon of salt equals 5,600 mg, with each teaspoon of salt containing 3,400 mg of chloride and 2,200 mg of sodium. Other dietary sources include all foods containing sodium chloride, as well as tomatoes, lettuce, olives, celery, rye, whole-grain foods, and seafood.
The AI for chloride in adults is 2,300 mg. Therefore, just ⅔ teaspoon of table salt per day is sufficient for chloride as well as sodium. The UL is set at 3,600 mg.2 Excess chloride in the blood is rare with no characteristic signs or symptoms.
Low dietary intake of chloride and more often diarrhea can cause low blood levels of chloride. Symptoms typically are similar to those of hyponatremia and include weakness, nausea, and headache.
Potassium
Potassium is the most abundant positively charged ion inside of cells. The majority of potassium (90%) exists in intracellular fluid, with about 10% in extracellular fluid, and only 1% in blood plasma. As with sodium, potassium levels in the blood are strictly regulated. The hormone aldosterone is what primarily controls potassium levels, but other hormones (such as insulin) also play a role. When potassium levels in the blood increase, the adrenal glands release aldosterone. Aldosterone acts on the collecting ducts of kidneys, where it stimulates an increase in the number of sodium-potassium pumps. Sodium is then reabsorbed and more potassium is excreted. Because potassium is required for maintaining sodium levels, and hence fluid balance, about 200 mg of potassium are lost from the body every day.
Nerve impulses involve not only sodium, but also potassium. A nerve impulse moves along a nerve via the movement of sodium ions into the cell. To end the impulse, potassium ions rush out of the nerve cell, thereby decreasing the positive charge inside the nerve cell. This diminishes the stimulus. To restore the original concentrations of ions between the intracellular and extracellular fluid, the sodium-potassium pump transfers sodium ions out in exchange for potassium ions in. On completion of the restored ion concentrations, a nerve cell is now ready to receive the next impulse. Similarly, in muscle cells potassium is involved in restoring the normal membrane potential and ending the muscle contraction. Potassium is also involved in protein synthesis, energy metabolism, platelet function, and acts as a buffer in blood, thus playing a role in acid-base balance.
Fruits and vegetables that contain high amounts of potassium are spinach, lettuce, broccoli, peas, tomatoes, potatoes, bananas, apples, and apricots. Whole grains and seeds, certain fish (such as salmon, cod, and flounder), and meats also provide potassium. Greater than 90% of dietary potassium is absorbed in the small intestine. Although highly bioavailable, potassium is a very soluble mineral and easily lost during cooking and processing of foods. Fresh and frozen foods are better sources of potassium than canned or processed foods.
Table 14.2.2 Potassium Content of Various Foods5
| Food | Serving | Potassium (mg) | %DV |
| Apricots, dried | ½ c | 1,101 | 23 |
| Lentils, cooked | 1 c | 731 | 16 |
| Acorn squash, mashed | 1 c | 644 | 14 |
| Raisins | ½ c | 618 | 13 |
| Potato, baked | 1 medium | 610 | 13 |
| Kidney beans, canned | 1 c | 607 | 13 |
| Orange juice | 1 c | 496 | 11 |
| Banana | 1 medium | 422 | 9 |
| Milk (1%) | 1 c | 366 | 8 |
| Spinach, raw | 2 c | 334 | 7 |
The AI for potassium is 3,400 mg/day for men and 2,600 mg/day for women. There is no UL set for potassium.5 Please recall that DRIs are applicable only to healthy individuals. People with chronic kidney disease or those using certain medications may be at higher risk for hyperkalemia which is very high levels of potassium in the blood. Although hyperkalemia can be asymptomatic, severe cases can cause muscle weakness, paralysis, heart palpitations, paresthesias (a burning or prickling sensation in the extremities), and cardiac arrhythmias (irregular heart beats) that could be life threatening.
Insufficient potassium levels in the body (hypokalemia) can be caused by a low dietary intake of potassium or by high sodium intakes, but more commonly it results from medications that increase water excretion, mainly diuretics. The signs and symptoms of hypokalemia are related to the functions of potassium in nerve cells and consequently skeletal and smooth muscle contraction and include muscle weakness and cramps, constipation, and respiratory distress. Severe potassium depletion can cause the heart to have abnormal contractions and can even be fatal.
Calcium
Calcium is the most abundant mineral in the body and greater than 99% of it is stored in bone tissue. Although only 1% of the calcium in the human body is found in the blood and soft tissues, it is here that it performs the most critical functions. Blood calcium levels are rigorously controlled so that if blood levels drop the body will rapidly respond by stimulating bone resorption (i.e., bone degradation or breakdown), thereby releasing stored calcium into the blood. Thus, bone tissue sacrifices its stored calcium to maintain blood calcium levels. This is why bone health is dependent on the intake of dietary calcium and also why blood levels of calcium do not necessarily correspond to dietary intake.
Calcium plays a role in a number of different functions in the body, but the most well known calcium function is to build and strengthen bones and teeth. When bone tissue first forms during the modeling or remodeling process, it is unhardened, protein-rich osteoid tissue. Bone building cells called osteoblasts provide bone mineralization and hardening by directing the deposit of calcium phosphates (salts) on the protein matrix. The calcium salts typically make up about 65% of bone tissue. When your diet is calcium deficient, and blood calcium levels fall, osteoclasts break apart the mineral content of bone to release the calcium. This causes bone to become brittle and weak. Thus, increased calcium intake helps to increase the mineralized content of bone tissue. Greater mineralized bone tissue corresponds to a greater bone mineral density and to greater bone strength.
One of the most important reasons that the body wants to maintain blood calcium levels is that it plays a role in nerve impulse transmission by facilitating electrical impulse transmission from one nerve cell to another. Another is that calcium in muscle cells is essential for muscle contraction because the flow of calcium ions are needed for the muscle proteins (actin and myosin) to interact. Calcium is also essential in blood clotting by activating clotting factors to fix damaged tissue. Because your brain (nervous system) and heart (muscle) must work 24 hours per day, it is essential that blood calcium levels are maintained.
Summary of Calcium Functions:
- necessary for building strong bones and teeth
- needed for nerve impulse transmission throughout the body
- necessary for muscle contraction including contraction of the heart muscle
- essential for blood clotting and repair of damaged tissue
In addition to these, calcium has several other minor functions that are also critical for maintaining normal physiology. For example, without calcium, the hormone insulin could not be released from cells in the pancreas and glycogen could not be broken down in muscle cells and used to provide energy for muscle contraction.
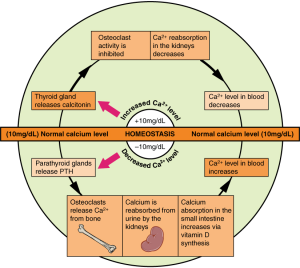
Because calcium performs such vital functions in the body, one’s blood calcium level is closely regulated by the hormones: parathyroid hormone (PTH), calcitriol, and calcitonin. If the blood calcium concentration drops too low, the parathyroid glands release PTH, which acts in several ways to increase blood calcium levels:
- PTH stimulates the activity of osteoclasts to release calcium from bone.
- PTH acts on the kidney to reduce the amount of calcium lost in the urine, returning more to circulation.
- PTH stimulates enzymes in the kidney that convert vitamin D to its active form, also called calcitriol. Activated vitamin D acts on the intestine to increase the absorption of calcium. Vitamin D also works together with PTH to stimulate release of calcium from the bone and reduce calcium loss in urine.
Once blood calcium levels are normal, PTH levels drop, turning off all of these mechanisms of increasing calcium. On the other hand, if blood calcium levels become too high, the thyroid gland releases calcitonin, which lowers blood calcium levels by increasing calcium excretion in the urine, preventing further absorption of calcium in the gut and by directly inhibiting bone resorption. Once blood calcium concentration returns to normal, calcitonin levels decrease.
Through these two opposing pathways—PTH and vitamin D for raising blood calcium and calcitonin for lowering blood calcium—the body can very effectively maintain blood calcium homeostasis. This system is dependent upon stored calcium in bone, which is sacrificed when needed to ensure adequate blood calcium. In the short-term, this isn’t a problem, because bone remodeling allows you to replace calcium in the bone. However, in the long term, inadequate dietary calcium means you continuously draw down the calcium stores in your bones, resulting in declining bone mineral density and increased risk of fracture.
Besides forming and maintaining strong bones and teeth, adequate calcium has been shown to have other health benefits for the body:
- Cancer. The National Cancer Institute reports that there is enough scientific evidence to conclude that higher intakes of calcium decrease colon cancer risk and may suppress the growth of polyps that often lead to cancer. Although higher calcium consumption protects against colon cancer, some studies have looked at the relationship between calcium and prostate cancer and found higher intakes may increase the risk for prostate cancer; however the data is inconsistent and more studies are needed to confirm any negative association.
- Blood pressure. Multiple studies provide clear evidence that higher calcium consumption reduces blood pressure. A review of 23 observational studies concluded that for every 100 mg of calcium consumed daily, systolic blood pressure is reduced 0.34 millimeter of mercury (mmHg) and diastolic blood pressure is decreased by 0.15 mmHg.6
- Cardiovascular health. There is emerging evidence that higher calcium intakes reduce other risk factors for cardiovascular disease, such as high cholesterol and obesity, but the scientific evidence is weak or inconclusive.
- Kidney stones. Another health benefit of a high calcium diet is that it blocks kidney stone formation. Calcium inhibits the absorption of oxalate, a chemical in plants such as parsley and spinach, which is associated with an increased risk for developing kidney stones. This protective effect of calcium on kidney stone formation occurs only when you obtain calcium from dietary sources. Calcium supplements may actually increase the risk for kidney stones in susceptible people.
In the typical American diet, calcium is obtained mostly from dairy products and foods fortified with calcium such as cereals, soy milk, and orange juice which also provide approximately ⅓ of the calcium RDA. Although the typical American diet relies mostly on dairy products for obtaining calcium, there are other good non-dairy sources of calcium, including some leafy green vegetables, legumes, soybeans (tofu, edamame, tempeh), and some seafood. Interestingly, the calcium in some vegetables such as kale, Brussel sprouts, and bok choy is better absorbed by the body than the calcium in dairy products where only about 30% of calcium is absorbed.
The greatest positive influence on calcium absorption comes from having an adequate intake of vitamin D. People deficient in vitamin D absorb less than 15% of calcium from the foods they eat. The hormone estrogen is another factor that enhances calcium bioavailability. Thus, as a woman ages and goes through menopause, during which estrogen levels fall, the amount of calcium absorbed decreases and the risk for bone disease increases. Some fibers, such as inulin, found in jicama, onions, and garlic, also promote calcium intestinal uptake.
However, there are also some chemicals that decrease calcium absorption including the oxalates in certain plants, the tannins in tea, phytates in nuts, seeds, and grains, and some fibers. Oxalates are found in high concentrations in spinach, parsley, cocoa, and beets. In general, the calcium bioavailability is inversely correlated to the oxalate content in foods. High-fiber, low-fat diets also decrease the amount of calcium absorbed, an effect likely related to how fiber and fat influence the amount of time food stays in the gut. Anything that causes diarrhea, including illness, medications, and certain symptoms related to old age, decreases the transit time of calcium in the gut and therefore decreases calcium absorption. As we get older, stomach acidity sometimes decreases, diarrhea occurs more often, kidney function is impaired, and vitamin D absorption and activation is compromised, all of which contribute to a decrease in calcium bioavailability.
Table 14.2.3 Calcium Content of Various Foods7
| Food | Serving | Calcium (mg) | %DV |
| Yogurt, low-fat | 1 c | 415 | 32 |
| Orange juice, calcium-fortified | 1 c | 349 | 29 |
| Mozzarella cheese, part skim | 1.5 oz | 333 | 26 |
| Sardines with bones, canned in oil | 3 oz | 325 | 25 |
| Cheddar cheese | 1.5 oz | 307 | 24 |
| Milk, nonfat | 1 c | 299 | 23 |
| Tofu, firm, made with calcium sulfate | ½ c | 253 | 19 |
| Cottage cheese (1%) | 1 c | 138 | 11 |
| Turnip greens, boiled | ½ c | 99 | 8 |
| Kale, cooked | 1 c | 94 | 7 |
The RDA for calcium for adults aged 19 to 50 is 1,000 mg/day. This number increases to 1,200 mg/day in women beginning at 51 years and men at 71 years. The UL for calcium, which applies to both food and supplements, is set at 2,500 mg/day for those between 19 to 50 years and decreases to 2,000 mg/day beginning at 51 years (for both men and women). Excessive calcium intake can lead to constipation, renal issues, vascular and soft tissue calcification, and kidney stones. However, consuming too much calcium from foods is rare and these are more likely to occur from use of supplements.7
Despite the wealth of evidence supporting the many health benefits of calcium (particularly bone health), the average American diet falls short of achieving the recommended dietary intakes of calcium. In fact, in females older than nine years of age, the average daily intake of calcium is only about 70% of the recommended intake. Here we will take a closer look at particular groups of people who may require extra calcium intake7:
- Adolescents. A calcium-deficient diet is common in teenagers (particularly girls) as their dairy consumption often drops considerably during adolescence.
- Amenorrheic women and the “female athlete triad.” Amenorrhea refers to the absence of a menstrual cycle. Women who fail to menstruate suffer from reduced estrogen levels, which can disrupt and have a negative impact on the calcium balance in their bodies. The female athlete triad is a combination of three conditions characterized by amenorrhea, disordered eating patterns, and osteopenia. For more information see Chapter 11.
- The elderly. As people age, calcium bioavailability is reduced, the kidneys lose their capacity to convert vitamin D to its most active form and are no longer efficient in retaining calcium, the skin is less effective at synthesizing vitamin D, there are changes in overall dietary patterns, and older people tend to get less exposure to sunlight.
- Post-menopausal women. Estrogen enhances calcium absorption. The decline in this hormone during and after menopause puts post-menopausal women especially at risk for calcium deficiency. Decreases in estrogen production are responsible for an increase in bone resorption and a decrease in calcium absorption.
- Lactose-intolerant people. Groups of people, such as those who are lactose intolerant, or who adhere to diets that avoid dairy products, may not have an adequate calcium intake.
- Vegans. Vegans typically absorb reduced amounts of calcium because their diets favor plant-based foods that contain oxalates and phytates.
*If you are lactose intolerant, have a milk allergy, and/or are a vegan, remember that there are several lactose-free dairy products on the market and many plant-based foods that have a good amount of calcium.
Osteoporosis
Osteoporosis is a disease where the bones become progressively more porous and fragile, resulting in much higher risk of fracture. It is considered a silent disease because one does not feel their bones weakening. Breaking a bone is often the first sign of osteoporosis. Other signs include loss of body height and curvature of the upper back.
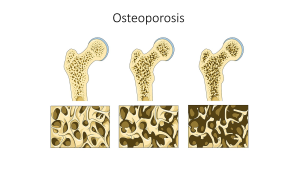
Approximately 54 million Americans have osteoporosis, with nearly 1 in 2 women and up to 1 in 4 men, aged 50 or older at risk of a osteoporotic bone fracture. The most common sites of fracture include hip, spine, or wrist. More than 20% of older adults who break a hip die within one year, either from complications of the break itself or the surgery to repair the break. Most will require long-term nursing home care.
In addition to low micronutrient intake, additional modifiable risk factors for osteoporosis include smoking, certain medications, history of eating disorder, excessive alcohol consumption, and physical inactivity.8 To prevent osteoporosis, consume adequate calcium and vitamin D throughout your lifetime, don’t smoke, and exercise regularly.
Calcium Supplements: Which One to Buy?
Many people choose to fulfill their daily calcium requirements by taking calcium supplements. Calcium supplements are sold primarily as calcium carbonate, calcium citrate, calcium lactate, and calcium phosphate, with elemental calcium contents of about 200 mg per pill. It is important to note that calcium carbonate requires an acidic environment in the stomach to be used effectively. Although this is not a problem for most people, it may be for those on medication to reduce stomach acid production or for the elderly who may have a reduced ability to secrete acid in the stomach. For these people, calcium citrate may be a better choice. Otherwise, calcium carbonate is the cheapest. The body is capable of absorbing approximately 30% of the calcium from these forms.
Beware of Lead
There is public health concern about the lead content of some brands of calcium supplements. Supplements derived from natural sources such as oyster shell, bone meal, and dolomite (a type of rock containing calcium magnesium carbonate) should be avoided as they are known to contain high amounts of lead. Because lead levels in supplements are not disclosed on labels, it is important to know that products not derived from oyster shell or other natural substances are generally low in lead content.
Diet, Supplements, and Chelated Supplements
In general, calcium supplements are less likely to provide health benefits linked to higher calcium intake than dietary sources of calcium. This is partly attributed to the fact that dietary sources of calcium supply additional nutrients with health-promoting activities. It is reported that chelated forms of calcium supplements are easier to absorb as the chelation process protects the calcium from oxalates and phytates that may bind with the calcium in the intestines. However, these are more expensive supplements and may only increase calcium absorption up to 10%. In people with low dietary intakes of calcium, calcium supplements have a negligible benefit on bone health in the absence of a vitamin D supplement.
Phosphorus
Phosphorus is present in our bodies as part of a chemical group called a phosphate group. These phosphate groups are essential as a structural component of cell membranes (as phospholipids), bones, teeth, DNA and RNA, energy production (ATP), and regulation of acid-base homeostasis. Phosphorus, however, is mostly associated with calcium as a part of the mineral structure of bones and teeth. While blood phosphorus levels are not controlled as strictly as calcium, the parathyroid hormone (PTH) stimulates renal excretion of phosphate so that it does not accumulate to toxic levels.
Table 14.2.4 Phosphorus Content of Various Foods9
| Food | Serving | Phosphorus (mg) | %DV |
| Yogurt, low-fat | 6 oz | 245 | 18 |
| Atlantic salmon, farmed, cooked | 3 oz | 214 | 17 |
| Chicken breast, roasted | 3 oz | 182 | 15 |
| Lentils, boiled | ½ c | 178 | 14 |
| Mozzarella cheese, part skim | 1.5 oz | 197 | 16 |
| Cashew nuts, roasted | 1 oz | 139 | 11 |
| Russet potato, baked | 1 medium | 123 | 10 |
| Peas, green, boiled | ½ c | 94 | 8 |
| Oatmeal, cooked with water | ½ c | 90 | 7 |
| Egg, hard boiled | 1 large | 86 | 7 |
The RDA for phosphorus for adults is 700 mg/day for men and women. The UL for phosphorus is set at 4,000 mg/day and applies to food and supplements. Excessive intake of phosphorus has been linked to cardiovascular, kidney, and bone adverse effects.9
In comparison to calcium, most Americans are not at risk for having a phosphate deficiency. It is present in many foods popular in the American diet including meat, fish, dairy products, processed foods, and beverages. It is also added to many foods because it acts as an emulsifying agent, prevents clumping, improves texture and taste, and extends shelf life. The average intake of phosphorus in US adults ranges between 1,000 and 1,500 mg per day, well above the RDA of 700 mg per day, but well below the UL.
Magnesium
Approximately 60% of magnesium in the human body is stored in the skeleton, making up about 1% of mineralized bone tissue. Observational studies link magnesium deficiency with an increased risk for osteoporosis. A magnesium deficient diet is associated with decreased levels of PTH and activation of vitamin D, which may lead to an impairment of bone remodeling. Only a few clinical trials have evaluated the effects of magnesium supplements on bone health and their results suggest some modest benefits on bone mineral density.
In addition to participating in bone maintenance, magnesium has several other functions in the body. In every reaction involving the cellular energy molecule (ATP), magnesium is required. Additionally, more than 300 enzymatic reactions require magnesium. It plays a role in the synthesis of protein, DNA, and RNA, is essential for nerve conduction, muscle contraction, and blood glucose control. Another health benefit of magnesium is that it may decrease blood pressure.
Magnesium is part of the green pigment, chlorophyll, which is vital for photosynthesis in plants; therefore, green leafy vegetables are a good dietary source for magnesium. Magnesium is also found in high concentrations in fish, dairy products, meats, whole grains, and nuts. Additionally chocolate, coffee, and hard water contain a good amount of magnesium. Typically, Western diets lean toward low fish intake and an unbalanced consumption of refined grains over whole grains. Therefore, most people in America do not meet the RDA for magnesium in their diets.
Table 14.2.5 Magnesium Content of Various Foods10
| Food | Serving | Magnesium (mg) | %DV |
| Almonds, roasted | 1 oz | 80 | 19 |
| Spinach, boiled | ½ c | 78 | 19 |
| Cashew nuts, roasted | 1 oz | 74 | 18 |
| Soymilk | 1 c | 61 | 15 |
| Black beans, cooked | ½ c | 60 | 14 |
| Edamame, shelled, cooked | ½ c | 50 | 12 |
| Peanut butter, creamy | 2 tbsp | 49 | 12 |
| Potato, baked | 3.5 oz | 43 | 10 |
| Banana | 1 medium | 32 | 8 |
| Avocado | ½ c | 22 | 5 |
The RDA for magnesium for adults age 19 to 30 is 400 mg/day for men and 310 mg/day for women. After age 30, this increases to 420 mg/day for men and 320 mg/day for women. The UL for magnesium is set at 350 mg/day and applies only to supplements (as this amount is is more than the RDA for women). Too much magnesium from food does not pose a health risk in healthy individuals as the kidneys eliminate excess amounts in the urine. Symptoms of excessive magnesium supplementation can include hypotension (low blood pressure), nausea, vomiting, facial flushing, and lethargy before progressing to muscle weakness, difficulty breathing, irregular heartbeat, and cardiac arrest.10
Magnesium deficiency due to low dietary intake is rare in healthy people, because the kidneys can decrease urinary excretion of this mineral when intake is inadequate. However those at greater risk of magnesium deficiency include people with type 2 diabetes, gastrointestinal diseases like Crohn’s and celiac, chronic alcoholism, and older adults. Symptoms may include decreased appetite, nausea, vomiting, fatigue, and weakness. If extreme, it can cause personality changes, muscle cramps, numbness, tingling, seizures, and an abnormal heart rhythm.10
Sulfur
Sulfur is incorporated into protein structures in the body. Amino acids, methionine and cysteine, contain sulfur which are essential for the antioxidant enzyme glutathione peroxidase. Some vitamins like thiamin and biotin also contain sulfur which are important in regulating acidity in the body. Sulfur is a major mineral with no recommended intake or deficiencies when protein needs are met. Sulfur is mostly consumed as a part of dietary proteins and sulfur containing vitamins.
14.3 Trace Minerals
Trace minerals (sometimes called Minor minerals) are classified as minerals required in the diet each day in smaller amounts, specifically 100 mg or less. These include iron, copper, zinc, selenium, iodine, chromium, fluoride, manganese, and molybdenum. Although trace minerals are needed in smaller amounts it is important to remember that a deficiency in a trace mineral can be just as detrimental to your health as a major mineral deficiency.
Iron
Red blood cells contain the oxygen-carrier protein hemoglobin. It is composed of four globular peptides, each containing a heme complex. In the center of each heme, lies iron. Iron is needed for the production of other iron-containing proteins such as myoglobin. Myoglobin is the oxygen-carrier protein found in the muscle tissues (myo = muscle) that enhances the amount of available oxygen for muscle contraction. Iron is also a key component of hundreds of metabolic enzymes. Many of the proteins of the electron transport chain contain iron-sulfur clusters involved in the transfer of high energy electrons and ultimately ATP synthesis. Iron is also involved in numerous metabolic reactions that take place mainly in the liver and detoxify harmful substances. Moreover, iron is required for DNA synthesis. The great majority of iron used in the body is recycled from the continuous breakdown of red blood cells.
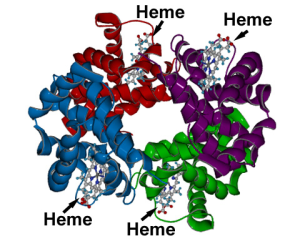
One of the most well known functions of hemoglobin, and specifically the iron within each heme complex, is that it binds to oxygen in the capillaries of the lungs and transports it to cells where the oxygen is released. If iron levels are low, hemoglobin is not synthesized in sufficient amounts and the oxygen-carrying capacity of red blood cells is reduced, resulting in anemia. When iron levels are low in the diet the small intestine more efficiently absorbs iron in an attempt to compensate for the low dietary intake, but this process cannot make up for the excessive loss of iron that occurs with chronic low intake or blood loss. When blood cells are decommissioned for use, the body recycles the iron back to the bone marrow where red blood cells are made. The body stores some iron in the bone marrow, liver, spleen, and skeletal muscle. A relatively small amount of iron is excreted when cells lining the small intestine and skin cells die and in blood loss, including menstrual bleeding. The lost iron must be replaced from dietary sources.
Table 14.3.1 Enhancers and Inhibitors of Iron Absorption
| Enhancer | Inhibitor |
| Meat | Phosphate |
| Fish | Calcium |
| Poultry | Tea |
| Seafood | Coffee |
| Stomach acid | High doses of minerals (antacids) |
| Vitamin C | High fiber |
| Phytates | |
| Oxalates | |
| Polyphenols |
There are two types of iron in foods: heme iron and non-heme iron. The bioavailability of iron is highly dependent on dietary sources. In animal-based foods about 40% of iron is bound to hemoglobin, and this heme iron is more bioavailable than non-heme iron. The other 60% of iron in animal based foods is non-heme, which is also the only iron source in plant-based foods. Bioavailability of iron is inhibited by substances some plants contain (such as phytate, oxalates, tannins, and polyphenols). However, eating iron-containing foods along with fruits and vegetables rich in vitamin C markedly increases iron absorption. Common enhancers and inhibitors of iron absorption are listed in Table 14.3.1.
Table 14.3.2 Iron Content of Various Foods11
| Food | Serving | Iron (mg) | %DV |
| Breakfast cereal, enriched | 1 serving | 18 | 100 |
| Eastern oysters, cooked | 3 oz | 8 | 44 |
| White beans, canned | 1 c | 8 | 44 |
| Dark chocolate, 45-69% cacao | 3 oz | 7 | 39 |
| Beef liver, pan fried | 3 oz | 5 | 28 |
| Lentils, boiled and drained | ½ c | 3 | 17 |
| Spinach, boiled | ½ c | 3 | 17 |
| Tofu, firm | ½ c | 3 | 17 |
| Kidney beans, canned | ½ c | 2 | 11 |
| Atlantic sardines, canned in oil | 3 oz | 2 | 11 |
The RDA for iron is 8 mg/day for men and 18 mg/day for women. The RDA for vegetarians is 1.8 times higher (or 14 mg/day for men or 32 mg/day for women) than for people who eat meat. The UL for iron is set at 45 mg/day and applies to both food and supplements.11 The body excretes little iron and therefore the potential for accumulation in tissues and organs is considerable. Iron accumulation in certain tissues and organs can cause a host of health problems in children and adults including extreme fatigue, joint pain, and severe liver and heart toxicity. In children, death has occurred from ingesting as little as 200 mg of iron and therefore it is critical to keep iron supplements out of children’s reach. Hemochromatosis is a hereditary disease resulting from a genetic mutation that leads to abnormal iron metabolism and an accumulation of iron in certain tissues such as the liver, pancreas, and heart. The signs and symptoms of hemochromatosis are similar to those of iron overload in tissues caused by a high intake of iron, but are often more severe and can include liver cirrhosis, cancer, heart disease, and impaired pancreatic function. It is usually diagnosed in middle age in men. In women it is usually not diagnosed until after menopause because, prior to that, monthly menstruation helps to mitigate the iron accumulation in tissues.
Iron Deficiency Anemia (IDA)
Iron deficiency anemia (IDA) is a condition that develops from having insufficient iron levels in the body resulting in fewer and smaller red blood cells containing lower amounts of hemoglobin. Regardless of the cause (be it from low dietary intake of iron or via excessive blood loss), IDA has the following signs and symptoms, which are linked to the essential functions of iron in energy metabolism and blood health:
- Fatigue
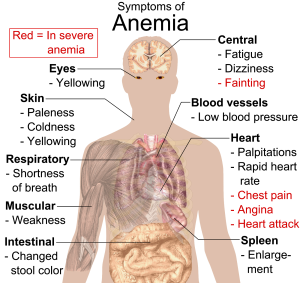
Figure 14.3.2 Symptoms of IDA - Weakness
- Pale skin
- Shortness of breath
- Dizziness
- Swollen, sore tongue
- Abnormal heart rate
IDA is diagnosed from these characteristic signs and symptoms and confirmed with simple blood tests that count red blood cells and determine hemoglobin and iron content in blood. Anemia is most often treated with iron supplements and increased consumption of iron rich foods. Iron supplements have some adverse side effects including nausea, constipation, diarrhea, vomiting, and abdominal pain. Reducing the dose at first and then gradually increasing to the full dose often minimizes the side effects of iron supplements. Avoiding foods and beverages high in phytates and also tea (which contains tannic acid and polyphenols, both of which impair iron absorption) is important for people who have IDA. Eating a dietary source of vitamin C at the same time as iron containing foods improves absorption of non-heme iron in the gut. Additionally, unknown compounds that likely reside in muscle tissue of meat, poultry, and fish increase iron absorption from both heme and nonheme sources (see Table 14.3.1).
The World Health Organization (WHO) reports that iron deficiency is the most common nutritional deficiency worldwide, affecting nearly 30% of the world population.12 The main causes of iron deficiency are parasitic worm infections in the gut causing excessive blood loss, and malaria, a parasitic disease causing the destruction of red blood cells. In the developed world, iron deficiency is more the result of dietary insufficiency and/or excessive blood loss occurring during menstruation or childbirth.
At-Risk Populations
Infants, children, adolescents, and women are the populations most at risk for IDA by all causes. Infants, children, and even teens require more iron because it is essential for growth. In these populations, iron deficiency (and eventually IDA) can also cause the following signs and symptoms: poor growth or failure to thrive, poor performance in school, and mental, motor, and behavioral disorders. Women who experience heavy menstrual bleeding or who are pregnant require more iron in the diet. One more high-risk group is the elderly. Both elderly men and women have a high incidence of anemia and the most common causes are dietary iron deficiency and chronic disease such as ulcer, inflammatory diseases, and cancer. Additionally, those who have recently suffered from traumatic blood loss, frequently donate blood, or take excessive antacids for heartburn need more iron in the diet.
Preventing IDA
In young children IDA can cause significant motor, mental, and behavioral abnormalities that are long-lasting. In the US, the high incidence of IDA in infants and children was a major public-health problem prior to the early 1970s, but now the incidence has been greatly reduced. This achievement was accomplished by implementing the screening of infants for IDA in the health sector as a common practice, advocating the fortification of infant formulas and cereals with iron, and distributing them in supplemental food programs, such as Women, Infants, and Children (WIC). Breastfeeding, iron supplementation, and delaying the introduction of cow’s milk for at least the first year of life are also encouraged. These practices were implemented across the socioeconomic spectrum and by the 1980s, IDA in infants had significantly declined. Other solutions had to be introduced in young children, who no longer were fed breast milk or fortified formulas and were instead consuming cow’s milk. These recommendations for parents include: providing a diet rich in sources of iron and vitamin C, limiting cow’s milk consumption to less than 24 oz per day, and providing a multivitamin containing iron.
Zinc
Zinc is a cofactor for over two hundred enzymes in the human body and plays a direct role in RNA, DNA, and protein synthesis. Zinc is also a cofactor for enzymes involved in energy metabolism.
A wide variety of foods contain zinc. While oysters contain more zinc per serving than any other food, red meat and poultry provide the majority of zinc in the American diet. Phytates, a compound present in whole grain breads, cereals, and legumes bind zinc and inhibit its absorption. Thus, bioavailability of zinc from grains and plant foods is lower than that from animal foods, although many plant-based foods are still good sources of zinc.13
Table 14.3.3 Zinc Content of Various Foods13
| Food | Serving | Zinc (mg) | %DV |
| Oysters, breaded, fried | 3 oz | 74 | 673 |
| Beef, chuck roast, braised | 3 oz | 7.0 | 64 |
| Crab, Alaska King, cooked | 3 oz | 6.5 | 59 |
| Lobster, cooked | 3 oz | 3.4 | 31 |
| Pork loin chop, cooked | 3 oz | 2.9 | 26 |
| Baked beans, vegetarian, canned | ½ c | 2.9 | 26 |
| Pumpkin seeds | 1 oz | 2.2 | 20 |
| Cashews, roasted | 1 oz | 1.6 | 15 |
| Swiss cheese | 1 oz | 1.1 | 11 |
| Milk, low-fat | 1 c | 1.0 | 9 |
The RDA for zinc is 11 mg/day for men and 8 mg/day for women. The UL for zinc is set at 40 mg/day and applies to both food and supplements. Signs and symptoms of zinc toxicity include nausea, vomiting, loss of appetite, abdominal cramps, diarrhea, and headaches.13
As the result of its prominent roles in anabolic and energy metabolism, a zinc deficiency in infants and children stunts growth. In adults, severe zinc deficiency can cause hair loss, diarrhea, skin sores, loss of appetite, and weight loss. Additionally, since zinc is a required cofactor for an enzyme that synthesizes the heme portion of hemoglobin, severely deficient zinc diets can result in anemia.
Iodine
Iodine is essential for the synthesis of thyroid hormone, which regulates basal metabolism, growth, and development.
The mineral content of foods is greatly affected by the soil from which it grew, and thus geographic location is the primary determinant of the mineral content of foods. For instance, iodine comes mostly from seawater so the greater the distance from the sea the lesser the iodine content in the soil. Seafood including seaweed (such as kelp, nori, kombu, and wakame) are some of the best food sources of iodine, but it is highly variable in its content. Since 1924, most salt in the US has been fortified with iodine. Iodized salt, dairy products, especially milk, and grains are the major contributors of iodine to the American diet.14
Table 14.3.4 Iodine Content of Various Foods14
| Food | Serving | Iodine (mcg) | %DV |
| Seaweed, sheet | 1 g | 16-2,984 | 11-1,989 |
| Cod | 3 oz | 99 | 66 |
| Plain yogurt, low-fat | 1 c | 75 | 50 |
| Iodized salt (¼ tsp) | 1.5 g | 71 | 47 |
| Milk, reduced-fat | 1 c | 56 | 37 |
| White bread | 2 slices | 45 | 30 |
| Ice cream, chocolate | ½ c | 30 | 20 |
| Egg | 1 large | 24 | 16 |
| Tuna, canned in oil | 3 oz | 17 | 11 |
| Prunes, dried | 5 each | 13 | 9 |
The RDA for iodine is 150 mcg/day for men and women. The UL for iodine is set at 1,100 mcg/day and applies to both food and supplements. However, people rarely exceed the UL.14
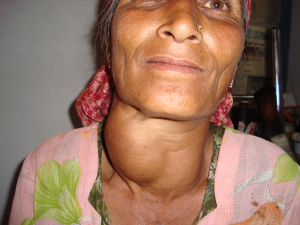
A deficiency of iodine leads to decreased production of thyroid hormones (T3 and T4), also called hypothyroidism, and eventually an enlargement of the thyroid tissue in an attempt to obtain more iodine, causing a goiter. Signs and symptoms of hypothyroidism include fatigue, sensitivity to cold, constipation, depression, and dry and itchy skin. The development of a goiter may often be the most visible sign of chronic iodine deficiency in adulthood, but the consequences of low levels of thyroid hormone in utero can be severe. Thyroid hormone plays a major role in brain development and growth and fetuses and infants with severe iodine deficiency develop a condition known as congenital iodine deficiency, characterized by significant physical and neurological impairment. The WHO estimates iodine deficiency affects over two billion people worldwide and is the number one cause of preventable brain damage worldwide.12,15
Copper
Copper, like iron, assists in electron transfer in the electron transport chain. Furthermore, copper is a cofactor of enzymes essential for iron absorption and transport. The other important function of copper is as an antioxidant. Symptoms of mild to moderate copper deficiency are rare. More severe copper deficiency can cause anemia from the lack of iron mobilization in the body for red blood cell synthesis. Other signs and symptoms include stunted growth in children and neurological problems, because copper is a cofactor for an enzyme that synthesizes myelin, which surrounds many nerves.
The richest sources of dietary copper include shellfish, seeds, and nuts.
Table 14.3.5 Copper Content of Various Foods16
| Food | Serving | Copper (mcg) | %DV |
| Beef liver, pan fried | 3 oz | 12,400 | 1,378 |
| Oysters, wild, cooked | 3 oz | 4,850 | 539 |
| Potato, cooked | 1 medium | 675 | 75 |
| Shiitake mushrooms, cooked | ½ c | 650 | 72 |
| Cashew nut, dry roasted | 1 oz | 629 | 70 |
| Crab, Dungeness, cooked | 3 oz | 624 | 69 |
| Sunflower seeds, toasted | ¼ c | 615 | 68 |
| Tofu, firm, raw | ½ c | 476 | 53 |
| Chickpeas | ½ c | 289 | 32 |
| Avocado | ½ c | 219 | 24 |
The AI for copper is 900 mcg/day for men and women. Copper deficiency is rare, as most Americans consume enough. The UL for copper is set at 10,000 mcg/day and applies to both food and supplements. Copper toxicity is rare in healthy individuals, although chronic exposure can result in liver damage and gastrointestinal symptoms, including abdominal pain, nausea, vomiting, and diarrhea.16
Selenium
Selenium is a cofactor of enzymes that release active thyroid hormone in cells and therefore low levels can cause similar signs and symptoms as iodine deficiency. The other important function of selenium is as an antioxidant.
Approximately 25 known proteins require selenium to function. Some are enzymes involved in detoxifying free radicals and include glutathione peroxidases and thioredoxin reductase. As an integral functioning part of these enzymes, selenium aids in the regeneration of glutathione and oxidized vitamin C. Selenium as part of glutathione peroxidase also protects lipids from free radicals, and, in doing so, spares vitamin E. This is just one example of how antioxidants work together to protect the body against free radical induced damage. Other functions of selenium containing proteins include protecting endothelial cells that line tissues, converting the inactive thyroid hormone to the active form in cells, and mediating inflammatory and immune system responses.
Seafoods and meat have some of the highest selenium content. Plants do not require selenium, so the content in fruits and vegetables is usually low. Grains and some nuts contain selenium when grown in selenium rich soil.
Table 14.3.6 Selenium Content of Various Foods17
| Food | Serving | Selenium (mcg) | %DV |
| Brazil nuts (6-8 nuts) | 1 oz | 544 | 989 |
| Yellowfin tuna, cooked | 3 oz | 92 | 167 |
| Halibut, cooked | 3 oz | 47 | 85 |
| Ham, roasted | 3 oz | 42 | 76 |
| Turkey, boneless, roasted | 3 oz | 31 | 56 |
| Cottage cheese (1%) | 1 c | 20 | 36 |
| Egg, hard boiled | 1 large | 15 | 27 |
| Bread, whole wheat | 1 slice | 13 | 24 |
| Vegetarian baked beans, canned | 1 c | 13 | 24 |
| Milk (1%) | 1 c | 8 | 15 |
The RDA for selenium is 55 mcg/day for men and women. Selenium at doses several thousand times the RDA can cause acute toxicity, and when ingested in gram quantities can be fatal. The UL for selenium is set for 400 mcg/day. Chronic intake of high levels of selenium can lead to selenosis, characterized by hair loss, nail brittleness, nausea, diarrhea, and lesions of the skin and nervous system.17
Chromium
The functioning of chromium in the body is less understood than that of most other minerals. It enhances the actions of insulin and plays a role in carbohydrate, fat, and protein metabolism. Currently, the results of scientific studies evaluating the usefulness of chromium supplementation in preventing and treating type 2 diabetes are largely inconclusive. More research is needed to better determine if chromium is helpful in treating certain chronic diseases and, if so, at what doses.
Meat and whole grain products, as well as some fruits, vegetables, and spices are relatively good sources. In contrast, foods high in simple sugars tend to be lower in chromium.
Table 14.3.7 Chromium Content of Various Foods18
| Food | Serving | Chromium (mcg) | %DV |
| Broccoli | ½ c | 11 | 31 |
| Grape juice | 1 c | 8 | 23 |
| English muffin, whole wheat | 1 | 4 | 11 |
| Potatoes, mashed | 1 c | 3 | 9 |
| Orange juice | 1 c | 2 | 6 |
| Turkey breast | 3 oz | 2 | 6 |
| Bread, whole wheat | 2 slices | 2 | 6 |
| Apple | 1 medium | 1 | 3 |
| Banana | 1 medium | 1 | 3 |
| Green beans | ½ c | 1 | 3 |
The AI for chromium is 35 mcg/day for adult males and 25 mcg/day for adult females. There is insufficient evidence to establish an UL for chromium.
Manganese
Manganese is a cofactor for enzymes that are required for carbohydrate and cholesterol metabolism, bone formation, and the synthesis of urea.
The best food sources of manganese in the diets of US adults include grains, nuts, legumes, and vegetables.
Table 14.3.8 Manganese Content of Various Foods19
| Food | Serving | Manganese (mcg) | %DV |
| Mussels, blue, cooked | 3 oz | 5.8 | 252 |
| Hazelnuts, roasted | 1 oz | 1.6 | 70 |
| Pecans, roasted | 1 oz | 1.1 | 48 |
| Brown rice, cooked | ½ c | 1.1 | 48 |
| Oysters, Pacific, cooked | 3 oz | 1.0 | 43 |
| Clams, cooked | 3 oz | 0.9 | 39 |
| Chickpeas, cooked | ½ c | 0.9 | 39 |
| Spinach, boiled | ½ c | 0.8 | 35 |
| Pineapple, chunks | ½ c | 0.8 | 35 |
| Bread, whole wheat | 1 slice | 0.7 | 30 |
The AI for manganese is 2.3 mg/day for men and 1.8 mg/day for women. Manganese deficiency is uncommon. The UL for manganese is 11 mg, however there is little evidence suggesting toxicity from high dietary manganese intakes.19
Molybdenum
Molybdenum acts as a cofactor for four enzymes necessary for the metabolism of sulfur-containing amino acids and nitrogen-containing compounds found in DNA and RNA. It also plays a role in the breakdown of drugs and toxic substances that enter the body.20
Legumes, whole grains, and nuts are some of the richest sources of molybdenum.
Table 14.3.9 Molybdenum Content of Various Foods20
| Food | Serving | Molybdenum (mcg) | %DV |
| Black eyed peas, boiled | ½ c | 288 | 640 |
| Lima beans, boiled | ½ c | 104 | 231 |
| Yogurt (2%), plain | 1 c | 26 | 58 |
| Milk (2%) | 1 c | 22 | 49 |
| Potato, baked | 1 medium | 16 | 36 |
| Banana | 1 medium | 15 | 33 |
| Bread, whole wheat | 1 slice | 12 | 27 |
| Peanuts, roasted | 1 oz | 11 | 24 |
| Egg, soft boiled | 1 large | 9 | 20 |
| Spinach, boiled | ½ c | 8 | 18 |
The RDA for molybdenum is 45 mcg/day for adult men and women. The UL for molybdenum is 2,000 mcg, although toxicity is rare as it is rapidly excreted in urine.20
Fluoride
Fluoride is known mostly as the mineral that combats tooth decay. It assists in tooth and bone development and maintenance. Fluoride combats tooth decay via three mechanisms:
- Blocking acid formation by bacteria
- Preventing demineralization of teeth
- Enhancing remineralization of destroyed enamel
Fluoride was first added to drinking water in 1945 in Grand Rapids, Michigan. Now over 60% of the US population consumes fluoridated drinking water. The Centers for Disease Control and Prevention (CDC) estimates that that fluoridation of water prevents, on average, 25% of cavities in children and adults. Community water fluoridation is recommended by nearly all public health, medical, and dental organizations. It is recommended by the American Dental Association, American Academy of Pediatrics, US Public Health Service, and WHO. The CDC considers water fluoridation one of the ten great public health achievements in the twentieth century.21
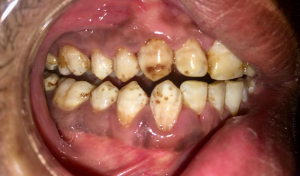
The optimal fluoride concentration in water to prevent tooth decay ranges between 0.7-1.2 mg per liter. Exposure to fluoride at 3-5 times this concentration before age 8 and the growth of permanent teeth can cause fluorosis, which is the mottling and discoloring of the teeth. When brushing the teeth of children younger than 2 years, it is recommended to use a child-sized soft bristled toothbrush and fluoridated toothpaste the size of a grain of rice. Try to ensure that the child does not swallow the toothpaste or any other fluoridated product (e.g., mouthwash). In some circumstances, based on the level of fluoride in community drinking water and other sources of fluoride, you may choose a non-fluoridated toothpaste. Check with your dentist. When assessing the risks and benefits, determine if the child may be at high risk for tooth decay because of factors such as poor hygiene, poor diet, or history of decay in the child, and in their siblings or parents. Fluoride supplements should be considered carefully.22
Greater than 70% of a person’s fluoride comes from drinking fluoridated water when they live in a community that fluoridates the drinking water. Other beverages with a high amount of fluoride include teas and grape juice. Solid foods do not contain a large amount of fluoride. Fluoride content in foods depends on whether it was grown in soils and water that contained fluoride or cooked with fluoridated water. Canned meats and fish that contain bones do contain some fluoride.
The AI for fluoride is 4 mg/day for men and 3 mg/day for women. The UL for fluoride in adults is 10 mg, however the period of greatest concern for fluorosis occurs prior to the age of 8.23
Key Takeaways
- Minerals are inorganic micronutrients categorized as major or trace based on how much the body requires daily. Some like sodium, chloride, and potassium, are electrolytes which means they can separate into charged ions when dissolved.
- Sodium is a major mineral and the primary regulator of water balance. It also plays important roles in nerve transmission, muscle contraction, and nutrient absorption and reabsorption.
- Most sodium in the typical American diet comes from processed and prepared foods, and most Americans consume more than they need. High sodium intake has been linked to hypertension.
- Chloride is linked to sodium in salt. It aids in fluid balance by helping to maintain charge neutrality. Chloride channels also play a role in regulating fluid secretion, such as the flow of pancreatic juice into the small intestine and the flow of water into mucus.
- Potassium is the most abundant positively charged ion inside of cells, and similar to sodium, potassium levels in the blood are under strict regulatory control. Potassium is the exchanged cation for sodium and helps maintain fluid balance. It also plays roles in nerve transmission, muscle contraction, protein synthesis, energy metabolism, platelet function, and acid-base balance. The best sources of potassium are whole foods like vegetables, legumes, and fruits.
- Calcium is the most abundant mineral in the body as the structural portion of bones and teeth. It also plays roles in nerve transmission, muscle contraction, blood clotting. Blood calcium levels are closely regulated by PTH, calcitriol, and calcitonin. Good dietary sources of calcium are dairy products, legumes, leafy greens. Many Americans do not consume adequate dietary calcium.
- Phosphorus is part of DNA, RNA, bones, teeth, and is needed for energy production.
- Magnesium is also part of bones, and helps with energy reactions creating ATP.
- Sulfur is incorporated into protein structures in the body, found in the amino acids cysteine and methionine.
- Iron is used for carrying oxygen on red blood cells. Iron deficiency anemia is the most common nutrient deficiency in the world. Absorption of iron from foods is enhanced and inhibited by several different factors.
- Zinc is a cofactor in hundreds of enzymes and plays a direct role in RNA, DNA, and protein synthesis.
- Iodine is essential for the synthesis of thyroid hormone which regulates basal metabolism, growth, and development. Deficiency of iodine causes goiter. The most important source of iodine in the American diet is iodized salt.
- Other trace minerals are copper, selenium which works as an antioxidant, chromium, manganese, molybdenum, and fluoride.
Portions of this chapter were taken from OER Sources listed below:
Titchenal, A., Calabrese, A., Gibby, C., Revilla, M.K.F., & Meinke, W. (2018). Human Nutrition. University of Hawai’i at Manoa Food Science and Human Nutrition Program Open Textbook. https://pressbooks.oer.hawaii.edu
Additional References:
- American Heart Association. (2018, May 23). Sodium sources: Where does all that sodium come from? https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/sodium/sodium-sources
- Institute of Medicine Food and Nutrition Board. (2004). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. The National Academies Press.
- United States Food and Drug Administration. (2009). Food labeling guide. http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodLabelingNutrition/FoodLabelingGuide/ucm064911.htm.
- American Heart Association. (2012). Shaking the salt habit. http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/PreventionTreatmentofHighBloodPressure/Shaking-the-Salt-Habit_UCM_303241_Article.jsp
- Office of Dietary Supplements. (2020, June 3). Potassium. National Institute of Health. https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/
- Birkett, N. J. (1998). Comments on a meta-analysis of the relation between dietary calcium intake and blood pressure. American Journal of Epidemiology, 148(3), 223-28. http://aje.oxfordjournals.org/content/148/3/223.long
- Office of Dietary Supplements. (2020, March 26). Calcium. National Institute of Health. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- National Osteoporosis Foundation. (n.d.). What is osteoporosis and what causes it? https://www.nof.org/patients/what-is-osteoporosis/
- Office of Dietary Supplements. (2020, June 4). Phosphorus. National Institute of Health. https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/
- Office of Dietary Supplements. (2020, March 24). Magnesium. National Institute of Health. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- Office of Dietary Supplements. (2020, February 28). Iron. National Institute of Health. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
- World Health Organization. (n.d.). Micronutrient deficiencies. https://www.who.int/nutrition/topics/ida/en/
- Office of Dietary Supplements. (2020, March 6). Zinc. National Institute of Health. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
- Office of Dietary Supplements. (2020, May 1). Iodine. National Institute of Health. https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/
- Biban, B. G., & Lichiardopol, C. (2017). Iodine deficiency, still a global problem? Current Health Sciences Journal, 43(2), 103-111. https://doi:10.12865/CHSJ.43.02.01
- Office of Dietary Supplements. (2020, June 3). Copper. National Institute of Health. https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/
- Office of Dietary Supplements. (2020, March 11). Selenium. National Institute of Health. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Office of Dietary Supplements. (2020, February 27). Chromium. National Institute of Health. https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/
- Office of Dietary Supplements. (2020, June 3). Manganese. National Institute of Health. https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/
- Office of Dietary Supplements. (2020, June 3). Molybdenum. National Institute of Health. https://ods.od.nih.gov/factsheets/Molybdenum-HealthProfessional/
- Centers for Disease Control and Prevention. (2020, January 15). Community water fluoridation. https://www.cdc.gov/fluoridation/index.html
- Centers for Disease Control and Prevention. (2019, March 8). Fluorosis. https://www.cdc.gov/fluoridation/faqs/dental_fluorosis/index.htm
- Institute of Medicine. (1997, January 1). Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. The National Academies Press. http://www.iom.edu/Reports/1997/Dietary-Reference-Intakes-for-Calcium-Phosphorus-Magnesium-Vitamin-D-and-Fluoride.aspx.
Media Attributions
- 22FA462D-E57A-409C-B2D9-8210B0613EA2
- 8E615429-FCBF-45E7-8339-63D1CC403285
- 86F8C9BD-FEDC-4691-9535-E914A84EA924
- 4FDBF91F-E067-46EF-A58A-285803D8899E
- 17AD0B22-0649-4F76-91E0-661CA4356914
- 08BD04BC-6852-4BFF-B351-F012A00DB620
- F282EF41-05C4-4D51-AE81-7E20C83A0A97
- 0B943CEF-DB22-4B4F-BFF9-5BB9CF7F8BDD
- 1C5D04B9-EBC5-4819-AECC-FE5CF41DCCB1
- 32CBF7EF-2243-4440-8D96-AE1388C39BF9
The amount of a substance that is absorbed, transported, and subsequently used in the body
fat found directly beneath the skin
positively charged electrolytes
very low levels of sodium in the blood; can be life-threatening, usually caused by excessive water intake (water intoxication)
High levels of potassium in the blood which can be life threatening.
Low levels of potassium in the blood.
bone-building cells
bone crushing cells
Protein on red blood cells that transports oxygen
Oxygen-carrier protein in muscle
Form of iron that is most easily absorbed, found only in animal foods.
Form of iron not well absorbed that is found in both plant and animal foods
Condition caused by a genetic mutation that leads to abnormal iron metabolism. Iron accumulates in organs such as liver, pancreas, and heart and can cause serious symptoms such as impaired pancreatic and liver function.
Most common nutrient deficiency disease in the world, caused by inadequate iron levels in the body. Common symptoms are fatigue, weakness, pale skin, dizziness, and shortness of breath.
A non-protein chemical compound (often a mineral) that is required for some enzymes to function.
Decreased production of thyroid hormones, primarily T3 and T4.
Enlargement of the thyroid gland caused by a severe and chronic deficiency of iodine.
