8 Sensation and Perception
Learning Objectives
- Explain newborn and infant perceptual abilities
- Describe sensory changes during adulthood
Our sensory systems are crucial for our ability to interact with the world. Every sense functions at birth—newborns use all their senses to attend to everything and every person. Some of our senses are fully developed at birth, while others are not. Furthermore, not all animals have all their sensory abilities at birth, for instance, kittens do not have their sense of vision at birth.
We will begin this section by exploring how infants’ senses develop and how sensory systems like hearing and vision operate. Additionally, we will discuss how infants take in information through their senses and transform the data into meaningful information. While some sensory systems change throughout the lifespan, the most drastic changes in sensory functioning occur in late adulthood. We will examine the most common types of changes to the sensory systems that older adults experience and will discuss ways to slow and cope with these changes.
8.1 Sensation and Perception in Infancy
Throughout much of history, the newborn was considered a passive, disorganized being who possessed minimal abilities. William James, an early psychologist, had described the newborn’s world as “a blooming, buzzing confusion” (Shaffer, 1985). However, current research techniques have demonstrated just how developed the newborn is with especially organized sensory and perceptual abilities.
Vision
The womb is a dark environment void of visual stimulation. Consequently, vision is one of the most poorly developed senses at birth, and time is needed to build neural pathways between the eyes and the brain (American Optometric Association [AOA], 2019). Newborns typically cannot see further than 8 to 10 inches away from their faces (AOA, 2019). An 8-week-old’s vision is 20/300. This means an object 20 feet away from an infant has the same clarity as an object 300 feet away from an adult with normal vision. By 3-months visual acuity has sharpened to 20/200, which would allow them the see the letter E at the top of a standard eye chart (Hamer, 2016). As a result, the world initially looks blurry to young infants (Johnson & deHaan, 2015).
 Why is visual acuity so poor in the infant? The fovea, which is the central field of vision in the retina and allows us to see sharp detail, is not fully developed at birth and does not start to reach adult levels of development until 15 months (Li & Ding, 2017). Even by 45 months some of the sensory neurons (cones) of the fovea are still not fully grown. Can babies see color? Young infants can perceive color, but the colors need to be very pure forms of basic colors, such as vivid red or green rather than weaker pastel shades. Most studies report that babies can see the full spectrum of colors by five months of age (AOA, 2019).
Why is visual acuity so poor in the infant? The fovea, which is the central field of vision in the retina and allows us to see sharp detail, is not fully developed at birth and does not start to reach adult levels of development until 15 months (Li & Ding, 2017). Even by 45 months some of the sensory neurons (cones) of the fovea are still not fully grown. Can babies see color? Young infants can perceive color, but the colors need to be very pure forms of basic colors, such as vivid red or green rather than weaker pastel shades. Most studies report that babies can see the full spectrum of colors by five months of age (AOA, 2019).
Newborn infants prefer and orient to face-like stimuli more than they do other patterned stimuli (Farroni et al., 2005). They also prefer images of faces that are upright and not scrambled (Chien, 2011). Infants also quickly learn to distinguish the faces of their mothers from the faces of other women (Bartrip et al., 2001). When viewing a person’s face, one-month-olds fixate on the outer edges of the face rather than the eyes, nose, or mouth, but two-month-olds gaze more at the inner features, especially the eyes (Hainline, 1978). Researchers have examined the development of attention and tracking in the visual system and have found the following for young infants:
- One-month-olds have difficulty disengaging their attention and can spend several minutes fixedly gazing at a stimulus (Johnson & deHaan, 2015).
- Aslin (1981) found that when tracking an object visually, the eye movements of newborns and one-month-olds are not smooth but saccadic, that is step-like jerky movements. Aslin also found that eye movements lag behind an object’s motion. This means young infants do not anticipate the trajectory of the object. By two months of age, their eye movements are becoming smoother, but they still lag behind the motion of the object and will not achieve this until about three to four months of age (Johnson & deHaan, 2015).
- Newborns also orient more to the visual field toward the side of the head, than to the visual field on either side of the nose (Lewis et al., 1979). By two to three months, stimuli in both fields are now attended to equally (Johnson & deHaan, 2015).
Binocular vision, which requires input from both eyes, is evident around the third month and continues to develop during the first six months (Atkinson & Braddick, 2003). By six months infants can perceive depth perception in pictures as well (Sen et al., 2001). Infants who have experience crawling and exploring will pay greater attention to visual cues of depth and modify their actions accordingly (Berk, 2007).
Hearing
The infant’s sense of hearing is very keen at birth, and the ability to hear is evidenced as soon as the seventh month of prenatal development. Newborns prefer their mother’s voices over another female even if speaking the same material (DeCasper & Fifer, 1980). Additionally, they will register in utero specific information heard from their mother’s voice. DeCasper and Spence (1986) tested 16 infants (average age of 55.8 hours) whose mothers had previously read to them prenatally. The mothers read several passages to their fetuses, including the first 28 paragraphs of The Cat in the Hat, beginning when they were 7 months pregnant. The fetuses had been exposed to the stories an average of 67 times or 3.5 hours. When the experimental infants were tested, the target stories (previously heard) were more reinforcing than the novel story as measured by their rate of sucking. However, for control infants, the target stories were not more reinforcing than the novel story indicating that the experimental infants had heard them before.
An infant can distinguish between very similar sounds as early as one month after birth and can distinguish between a familiar and unfamiliar voice even earlier. Infants are especially sensitive to the frequencies of sounds in human speech and prefer the exaggeration of infant-directed speech, which will be discussed in a later chapter. Additionally, infants are innately ready to respond to the sounds of any language, but between six and nine months they show a preference for listening to their native language (Jusczyk et al., 1993). Their ability to distinguish the sounds that are not in the language around them diminishes rapidly (Cheour-Luhtanen et al., 1995).
Touch and Pain
Immediately after birth, a newborn is sensitive to touch and temperature, and is also highly sensitive to pain, responding with crying and cardiovascular responses (Balaban & Reisenauer, 2013).
Newborns who are circumcised, which is the surgical removal of the foreskin of the penis, without anesthesia experience pain as demonstrated by increased blood pressure, increased heart rate, decreased oxygen in the blood, and a surge of stress hormones (United States National Library of Medicine, 2016). Research has demonstrated that infants who were circumcised without anesthesia experienced more pain and fear during routine childhood vaccines. Fortunately, today many local painkillers are currently used during circumcision.
Taste and Smell
Studies of taste and smell demonstrate that babies respond with different facial expressions, suggesting that certain preferences are innate. Newborns can distinguish between sour, bitter, sweet, and salty flavors and show a preference for sweet flavors. Newborns also prefer the smell of their mothers. An infant only 6 days old is significantly more likely to turn toward its own mother’s breast pad than to the breast pad of another baby’s mother (Porter et al., 1992), and within hours of birth, an infant also shows a preference for the face of its mother (Bushnell, 2001; Bushnell et al., 1989).
Intermodality
Infants seem to be born with the ability to perceive the world in an intermodal way; that is, through stimulation from more than one sensory modality. For example, infants who sucked on a pacifier with either a smooth or textured surface preferred to look at a corresponding (smooth or textured) visual model of the pacifier. By 4 months, infants can match lip movements with speech sounds and can match other audiovisual events. Sensory processes are certainly affected by the infant’s developing motor abilities (Hyvärinen et al., 2014). Reaching, crawling, and other actions allow the infant to see, touch, and organize his or her experiences in new ways.
How Infants Are Tested
 Habituation procedures, that is measuring decreased responsiveness to a stimulus after repeated presentations, have increasingly been used to evaluate infants in studies of the development of perceptual and memory skills. Phelps (2005) describes a habituation procedure used when measuring the rate of the sucking reflex. Researchers first measure the initial baseline rate of sucking to a pacifier equipped with transducers that measure muscle contractions. Next, an auditory stimulus is presented, such as a human voice uttering a speech sound such as “da.” The rate of sucking will typically increase with the new sound but then decrease to baseline levels as “da” is repeatedly presented, showing habituation. If the sound “ma” was then presented, the rate of sucking would again increase, demonstrating that the infant can discriminate between these two stimuli.
Habituation procedures, that is measuring decreased responsiveness to a stimulus after repeated presentations, have increasingly been used to evaluate infants in studies of the development of perceptual and memory skills. Phelps (2005) describes a habituation procedure used when measuring the rate of the sucking reflex. Researchers first measure the initial baseline rate of sucking to a pacifier equipped with transducers that measure muscle contractions. Next, an auditory stimulus is presented, such as a human voice uttering a speech sound such as “da.” The rate of sucking will typically increase with the new sound but then decrease to baseline levels as “da” is repeatedly presented, showing habituation. If the sound “ma” was then presented, the rate of sucking would again increase, demonstrating that the infant can discriminate between these two stimuli.
Additionally, the speed or efficiency with which infants show habituation has been shown to predict outcomes in behaviors, such as language acquisition and verbal and nonverbal intelligence. Infants who show difficulty during habituation, or habituate at slower than normal rates, are at an increased risk for significant developmental delays. Infants with Down syndrome, teratogen-exposed infants, malnourished infants, and premature infants have all been studied. Researchers have found that at the age of 16 months, high-risk infants show rates of habituation comparable to newborn infants (Phelps, 2005).
8.2 Sensation & Perception in Adulthood
Vision
In late adulthood, all the senses show signs of decline, especially among the oldest-old. In the last chapter, you read about the visual changes that were beginning in middle adulthood, such as presbyopia, dry eyes, and problems seeing in dimmer light. By later adulthood, these changes are much more common. Three serious eye diseases are also more common in older adults: cataracts, macular degeneration, and glaucoma. Only the first can be effectively cured in most people.
Cataracts
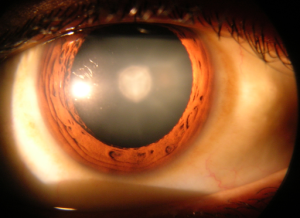
Cataracts are a clouding of the lens of the eye. The lens of the eye is made up of mostly water and protein. The protein is precisely arranged to keep the lens clear, but with age, some of the protein starts to clump. As more of the protein clumps together the clarity of the lens is reduced. While some adults in middle adulthood may show signs of cloudiness in the lens, the area affected is usually small enough not to interfere with vision. More people have problems with cataracts after age 60 (NIH, 2014) and by age 75, 70% of adults will have problems with cataracts (Boyd, 2014). Cataracts also cause a discoloration of the lens, tinting it more yellow and then brown, which can interfere with the ability to distinguish colors such as black, brown, dark blue, or dark purple.
Risk factors besides age include certain health problems such as diabetes, high blood pressure, and obesity, behavioral factors such as smoking, other environmental factors such as prolonged exposure to ultraviolet sunlight, previous trauma to the eye, long-term use of steroid medication, and a family history of cataracts (NEI, 2016a; Boyd, 2014). Cataracts are treated by removing and replacing the lens of the eye with a synthetic lens. In developed countries, such as the United States, cataracts can be easily treated with surgery. However, in developing countries, access to such operations are limited, making cataracts the leading cause of blindness in late adulthood in the least developed countries (Resnikoff et al., 2004).
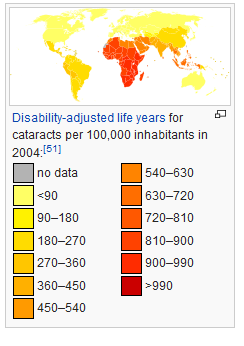 As shown in the image on the left, in areas of the world with limited medical treatment for cataracts, people are living more years with a serious disability. For example, of those living in the darkest red color on the map, more than 990 out of 100,00 people have a shortened lifespan due to the disability caused by cataracts.
As shown in the image on the left, in areas of the world with limited medical treatment for cataracts, people are living more years with a serious disability. For example, of those living in the darkest red color on the map, more than 990 out of 100,00 people have a shortened lifespan due to the disability caused by cataracts.
Macular Degeneration
Older adults are also more likely to develop age-related macular degeneration, which is the loss of clarity in the center field of vision, due to the deterioration of the macula, the center of the retina. Macular degeneration does not usually cause total vision loss, but the loss of the central field of vision can greatly impair day-to-day functioning. There are two types of macular degeneration: dry and wet. The dry type is the most common form and occurs when tiny pieces of a fatty protein called drusen form beneath the retina. Eventually the macular becomes thinner and stops working properly (Boyd, 2016). About 10% of people with macular degeneration have the wet type, which causes more damage to their central field of vision than the dry form. This form is caused by an abnormal development of blood vessels beneath the retina. These vessels may leak fluid or blood causing more rapid loss of vision than the dry form.
The risk factors for macular degeneration include smoking, which doubles your risk (NIH, 2015); race, as it is more common among Caucasians than African Americans or Hispanics/Latinos; high cholesterol; and a family history of macular degeneration (Boyd, 2016). At least 20 different genes have been related to this eye disease, but there is no simple genetic test to determine your risk, despite claims by some genetic testing companies (NIH, 2015). At present, there is no effective treatment for the dry type of macular degeneration. Some research suggests that some patients may benefit from a cocktail of certain antioxidant vitamins and minerals, but results are mixed at best. They are not a cure for the disease, nor will they restore the vision that has been lost. This “cocktail” can slow the progression of visual loss in some people (Boyd, 2016; NIH, 2015). For the wet type, medications that slow the growth of abnormal blood vessels and surgery, such as laser treatment to destroy the abnormal blood vessels, may be used. Unfortunately, only 25% of those with the wet version typically see improvement with these procedures (Boyd, 2016).
Glaucoma
A third vision problem that increases with age is glaucoma, which is the loss of peripheral vision, frequently due to a buildup of fluid in the eye that damages the optic nerve. As we age the pressure in the eye may increase causing damage to the optic nerve. The exterior of the optic nerve receives input from retinal cells on the periphery, and as glaucoma progresses more and more of the peripheral visual field deteriorates toward the central field of vision. In the advanced stages of glaucoma, a person can lose their sight entirely. Fortunately, glaucoma tends to progress slowly (NEI, 2016b).

Normal vision |
 |
 |
 |
Glaucoma is the most common cause of blindness in the U.S. (NEI, 2016b). African Americans over age 40, and everyone else over age 60, have a higher risk for glaucoma. Those with diabetes, and with a family history of glaucoma also have a higher risk (Owsley et al., 2015). There is no cure for glaucoma, but its rate of progression can be slowed, especially with early diagnosis. Routine eye exams to measure eye pressure and examination of the optic nerve can detect both the risk and presence of glaucoma (NEI, 2016b). Those with elevated eye pressure are given medicated eye drops. Reducing eye pressure lowers the risk of developing glaucoma or slowing its progression in those who already have it.
Hearing
As you read previously, our hearing declines both in terms of the frequencies of sound we can detect, and the intensity of sound needed to hear as we age. These changes continue in late adulthood. Almost 1 in 4 adults aged 65 to 74 and 1 in 2 aged 75 and older have disabling hearing loss (NIH, 2016).
Presbycusis is a common form of hearing loss in late adulthood that results in a gradual loss of hearing. It runs in families and affects hearing in both ears (NIA, 2015b). Older adults may also notice tinnitus, a ringing, hissing, or roaring sound in the ears. The exact cause of tinnitus is unknown, although it can be related to hypertension and allergies. It may come and go or persist and get worse over time (NIA, 2015b). The incidence of both presbycusis and tinnitus increases with age and males around the world have higher rates of both (McCormak et al., 2016).
Your auditory system has two jobs: To help you hear and to help you maintain balance. Your balance is controlled when the brain receives information from the shifting of hair cells in the inner ear about the position and orientation of the body. With age, the functionality of the inner ear declines, which can lead to problems with balance when sitting, standing, or moving (Martin, 2014).
Common Signs of Hearing Loss
- Trouble hearing while on the phone
- Has a hard time following conversations when two or more people are talking
- Asks people to repeat what they are saying frequently
- Needs to turn up the TV or device volume so loud that others complain
- Has as problem hearing because of background noise
- Believes that others seem to mumble
- Can’t understand when children or women are speaking
Information adapted from the NIA, 2015
Taste and Smell
Our sense of taste and smell is part of our chemical sensing system. Our sense of taste, or gustation, appears to age well. Normal taste occurs when molecules that are released by chewing food stimulate taste buds along the tongue, the roof of the mouth, and in the lining of the throat. These cells send messages to the brain, where specific tastes are identified. After age 50, we start to lose some of these sensory cells. Most people do not notice any changes in taste until their 60s (NIH: Senior Health, 2016). Given that the loss of taste buds is very gradual, even in late adulthood, many people are often surprised that their loss of taste is most likely the result of a loss of smell.
Our sense of smell, or olfaction, decreases with age, and problems with the sense of smell are more common in men than in women. Almost 1 in 4 males in their 60s have a disorder with the sense of smell, compared to 1 in 10 women (NIH: Senior Health, 2016). This loss of smell due to aging is called presbyosmia. Olfactory cells are located in a small area high in the nasal cavity. These cells are stimulated via two pathways: when we inhale through the nose, or via the connection between the nose and the throat when we chew and digest food. It is a problem with this second pathway that explains why some foods such as chocolate or coffee seem tasteless when we have a head cold. There are several types of loss of smell. Total loss of smell, or anosmia, is extremely rare.
Problems with our chemical senses can be linked to other serious medical conditions such as Parkinson’s, Alzheimer’s, or multiple sclerosis (NIH: Senior Health, 2016). Any sudden changes in sensory sensitivity should be checked out. Loss of smell can change a person’s diet, with either a loss of enjoyment of food and eating too little for balanced nutrition or adding sugar and salt to foods that are becoming blander to the palette.
Touch
Research has found that with age, people may experience reduced or changed sensations of vibration, cold, heat, pressure, and pain (Martin, 2014). Many of these changes are also consistent with a number of medical conditions that are more common among the elderly, such as diabetes. However, there are also changes in touch sensations among healthy older adults. The ability to detect changes in pressure has been shown to decline with age, with more pronounced losses during the 6th decade and diminishing further with advanced age (Bowden & McNelty, 2013). Yet, there is considerable variability, with almost 40% of the elderly showing sensitivity that is comparable to younger adults (Thornbury & Mistretta, 1981). However, the ability to detect the roughness/ smoothness or hardness/softness of an object shows no appreciable change with age (Bowden & McNulty, 2013). Those who show decreasing sensitivity to pressure, temperature, or pain are at risk for injury (Martin, 2014), as they can injure themselves without detecting it.
Pain
According to Molton and Terrill (2014), approximately 60%-75% of people over the age of 65 report at least some chronic pain, and this rate is even higher for those individuals living in nursing homes. Although the presence of pain increases with age, older adults are less sensitive to pain than younger adults (Harkins et al., 1986). Farrell (2012) looked at research studies that included neuroimaging techniques involving older people who were healthy and those who experienced a painful disorder. Results indicated that there were age-related decreases in brain volume in those structures involved in pain. Especially noteworthy were changes in the prefrontal cortex, brainstem, and hippocampus.
Women are more likely to report feeling pain than men (Tsang et al., 2008). Women have fewer opioid receptors in the brain, and women also receive less relief from opiate drugs (Garrett, 2015). Because pain serves as an important indicator that there is something wrong, a decreased sensitivity to pain in older adults is a concern because it can conceal illnesses or injuries requiring medical attention.
Chronic health problems, including arthritis, cancer, diabetes, joint pain, sciatica, and shingles are responsible for most of the pain felt by older adults (Molton & Terrill, 2014). Cancer is a special concern, especially “breakthrough pain” which is a severe pain that comes on quickly while a patient is already medicated with a long-acting painkiller. It can be very upsetting, and after one attack many people worry it will happen again. Some older individuals worry about developing an addiction to pain medication, but if the medicine is taken exactly as prescribed, addiction should not be a concern (NIH, 2015a). Lastly, side effects from pain medicine, including constipation, dry mouth, and drowsiness, may occur that can adversely affect the elder’s life.
Some older individuals put off going to the doctor because they think pain is just part of aging and nothing can help. Of course, this is not usually true. Managing pain is crucial to ensure feelings of well-being for the older adult. When chronic pain is not managed, the individual tends to restrict their movements for fear of feeling pain or injuring themselves further. This lack of activity will result in more restriction, further decreased participation, and greater disability (Jensen et al., 2011). A decline in physical activity because of pain is also associated with weight gain and obesity in adults (Strine et al., 2005). Additionally, sleep and mood disorders, such as depression, can occur (Moton & Terrill, 2014). Learning to cope effectively with pain is an important consideration in late adulthood and working with one’s primary physician or a pain specialist is recommended (NIH, 2015a).
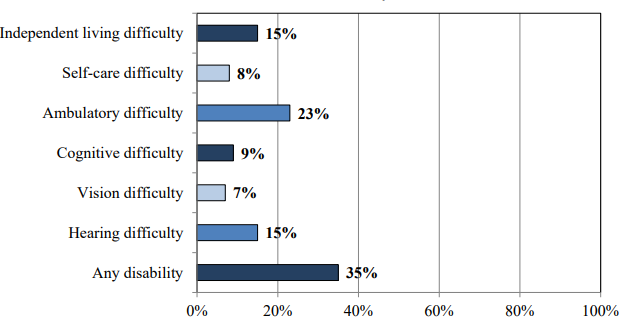
Of those 65 and older, 35% have a disability. The image below identifies the percentage of those who have a disability based on the type.
References
American Optometric Association. (2019). Infant vision: Birth to 24 months of age. https://www.aoa.org/patients-and-public/good-vision-throughout-life/childrens-vision/infant-vision-birth-to-24-months-of-age
Aslin, R. N. (1981). Development of smooth pursuit in human infants. In D. F. Fisher, R. A. Monty, & J.W. Senders (Eds.), Eye movements: Cognition and visual perception (pp. 31– 51). Erlbaum.
Atkinson, J., & Braddick, O. (2003). Neurobiological models of normal and abnormal visual development. In M. de Haan & M. H. Johnson (Eds.), The Cognitive Neuroscience of Development (pp.43–71). Psychology Press.
Balaban, M. T. & Reisenauer, C. D. (2013). Sensory development. In N. J. Salkind (Ed.), Encyclopedia of Human Development (pp. 1144-1147). Sage Publications.
Bartrip J, Morton J, & De Schonen S. (2001). Responses to mother’s face in 3-week to 5-month-old infants. British Journal of Developmental Psychology, 19, 219–232.
Berk, L. E. (2007). Development through the lifespan (4th ed.). Allyn and Bacon.
Bowden, J. L., & McNulty, P. A. (2013). Age-related changes in cutaneous sensation in the healthy human hand. Age (Dordrecht, Netherlands), 35(4), 1077-1089.
Boyd, K. (2014). What are cataracts? American Academy of Ophthalmology. http://www.aao.org/eyehealth/diseases/what-are-cataracts
Bushnell, I. W. R. (2001) Mother’s face recognition in newborn infants: Learning and memory. Infant Child Development, 10, 67-94.
Bushnell, I. W. R., Sai, F., Mullin, J. T. (1989). Neonatal recognition of mother’s face. British Journal of Developmental Psychology, 7, 3-15.
Cheour-Luhtanen, M., Alho. K., Kujala, T., Reinikainen, K., Renlund, M., Aaltonen, O., … & Näätänen. (1995). Mismatch negativity indicates vowel discrimination in newborns. Hearing Research, 82, 53–58.
Chien S. (2011). No more top-heavy bias: Infants and adults prefer upright faces but not top-heavy geometric or face-like patterns. Journal of Vision, 11(6):1–14.
DeCasper, A. J., & Fifer, W. P. (1980). Of human bonding: Newborns prefer their mother’s voices. Science, 208, 1174-1176.
DeCasper, A. J., & Spence, M. J. (1986). Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behavior and Development, 9, 133-150.
Farrell, M. J. (2012). Age-related changes in the structure and function of brain regions involved in pain processing. Pain Medication, 2, S37-43. doi: 10.1111/j.1526-4637.2011.01287.x.
Farroni, T., Johnson, M.H. Menon, E., Zulian, L. Faraguna, D., Csibra, G. (2005). Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proceedings of the National Academy of Sciences of the United States of America, 102(47), 17245-17250.
Garrett, B. (2015). Brain and behavior (4th ed.) Thousand Oaks, CA: Sage.
Hainline L. (1978). Developmental changes in visual scanning of face and nonface patterns by infants. Journal of Experimental Child Psychology, 25, 90–115.
Hamer, R. (2016). The visual world of infants. Scientific American, 104, 98-101.
Harkins, S. W., Price, D. D. & Martinelli, M. (1986). Effects of age on pain perception. Journal of Gerontology, 41, 58-63.
Hyvärinen, L., Walthes, R., Jacob, N., Nottingham Chaplin, K., & Leonhardt, M. (2014). Current understanding of what infants see. Current Ophthalmological Report, 2, 142-149. doi:10.1007/s40135-014-0056-2
Jensen, M. P., Moore, M. R., Bockow, T. B., Ehde, D. M., & Engel, J. M. (2011). Psychosocial factors and adjustment to persistent pain in persons with physical disabilities: A systematic review. Archives of Physical Medicine & Rehabilitation, 92, 146–160. doi:10.1016/j.apmr.2010 .09.021
Johnson, M. H., & deHaan, M. (2015). Developmental cognitive neuroscience: An introduction. Wiley & Sons.
Jusczyk, P.W., Cutler, A., & Redanz, N.J. (1993). Infants’ preference for the predominant stress patterns of English words. Child Development, 64, 675–687.
Lewis, T. L., Maurer, D., & Milewski, A. (1979). The development of nasal detection in young infants. Investigative Ophthalmology and Visual Science Supplement, 19, 271.
Li, Y., & Ding, Y. (2017). Human visual development. In Y. Liu., & W. Chen (Eds.), Pediatric lens diseases (pp. 11-20). Springer.
Martin, L. J. (2014). Age changes in the senses. MedlinePlus. https://medlineplus.gov/ency/article/004013.htm
McCormak A., Edmondson-Jones M., Somerset S., & Hall D. (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research, 337, 70-79.
Molton, I. R., & Terrill, A. L. (2014). Overview of persistent pain in older adults. American Psychologist, 69(2), 197-207.
National Eye Institute. (2016a). Cataract. https://nei.nih.gov/health/cataract/
National Eye Institute. (2016b). Glaucoma. https://nei.nih.gov/glaucoma/
National Institute on Aging. (2015a). Global Health and Aging. https://www.nia.nih.gov/research/publication/global-health-and-aging/living-longer
National Institute on Aging. (2015b). Hearing loss. https://www.nia.nih.gov/health/publication/hearingloss
National Institutes of Health. (2014). Cataracts. https://medlineplus.gov/cataract.html
National Institutes of Health. (2015). Macular degeneration. https://medlineplus.gov/maculardegeneration.html
National Institutes of Health. (2016). Quick statistics about hearing. https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing
National Institutes of Health: Senior Health (2016). Problems with smell. https://nihseniorhealth.gov/problemswithsmell/aboutproblemswithsmell/01.html
Owsley, C., Rhodes, L. A., McGwin Jr., G., Mennemeyer, S. T., Bregantini, M., Patel, N., … Girkin, C.A. (2015). Eye care quality and accessibility improvement in the community (EQUALITY) for adults at risk for glaucoma: Study rationale and design. International Journal for Equity in Health, 14, 1-14. doi: 10:1186/s12939-015-0213-8
Phelps, B. J. (2005). Habituation. In N. J. Salkind (Ed.), Encyclopedia of human development (pp. 597-600). Sage Publications.
Porter, R. H., Makin, J. W., Davis, L. M., Christensen, K. (1992). Responsiveness of infants to olfactory cues from lactating females. Infant Behavior and Development, 15, 85-93.
Resnikov, S., Pascolini, D., Mariotti, S. P., & Pokharel, G. P. (2004). Global data on visual impairment in the year 2002. Bulletin of the World Health Organization, 82, 844-851.
Sen, M. G., Yonas, A., & Knill, D. C. (2001). Development of infants’ sensitivity to surface contour information for spatial layout. Perception, 30, 167-176.
Shaffer, D. R. (1985). Developmental psychology: Theory, research, and applications. Wadsworth, Inc.
Strine, T. W., Hootman, J. M., Chapman, D. P., Okoro, C. A., & Balluz, L. (2005). Health-related quality of life, health risk behaviors, and disability among adults with pain-related activity difficulty. American Journal of Public Health, 95, 2042–2048. doi: 10.2105/AJPH.2005 .066225
Thornbury, J. M., & Mistretta, C. M. (1981). Tactile sensitivity as a function of age. Journal of Gerontology, 36(1), 34-39.
Tsang, A., Von Korff, M., Lee, S., Alonso, J., Karam, E., Angermeyer, M. C., . . .Watanabe, M. (2008). Common persistent pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression anxiety disorders. The Journal of Pain, 9, 883–891. doi: 10.1016/j.jpain.2008.05.005
United States National Library of Medicine. (2016). Circumcision. https://medlineplus.gov/circumcision.html
Media Attributions
- mother-84628_1280 © PublicDomainPictures is licensed under a CC BY-SA (Attribution ShareAlike) license
- cake-2675870_1280 © timkraaijvanger is licensed under a CC BY-SA (Attribution ShareAlike) license
- 512px-Cataract_in_human_eye © Rakesh Ahuja, MD is licensed under a CC BY-SA (Attribution ShareAlike) license
