2 Genetics of Sex
STUDENT LEARNING OUTCOMES
At the end of this chapter you should be able to:
- Explain the role of sex chromosomes in genetic sex determination
- Compare and contrast different modes of sex determination
- Discuss what a“master switch” is for sex determination
- Recall how the X chromosome is distinct from the Y chromosome
- Describe how ESD is different from GSD
- Contrast the dosage compensation mechanisms
- Explain the process of gonadal development
- Discuss the complex nature of sex determination
- Describe DSDs and explain how they can occur
Nettie Maria Stevens discovers sex chromosomes

Nettie Maria Stevens (pictured on the right) has the distinction of being the first woman geneticist in the male-dominated world of the early 1900s. Through persistence and hard work, she discovered sex chromosomes and posited how they fit with Mendelian principles of heredity. Studying insects as a student first at Stanford and later as an Assistant Professor at Bryn Mawr, Stevens was able to determine that females have two X chromosomes, and males only one X chromosome. Stevens was also the first to identify that males had a single, small chromosome (the Y chromosome) and recognize its association with male development (Stevens 1905). She was able to complete an amazing amount of work in a short amount of time especially given that most doors were closed to women in science at that time. Thomas Hunt Morgan commented on her excellence in a letter of recommendation for her saying: “of the graduate students that I have had during the last twelve years I have had no one that was as capable and independent in research work as Miss Stevens . . .” (Brush 1978). Stevens was able to earn her Ph.D. at age 42 at Bryn Mawr, only after working long enough to save up for tuition as a teacher/librarian and proving herself through numerous research assistant positions from Stanford’s Hopkins Seaside Laboratory to the University of Würzburg, working with Theodor Boveri.
Steven’s death at age 50 from breast cancer tragically truncated her career. Carey et al., (2022) provide a review of her accomplishments noting that when her paper was published another male scientist (E. B. Wilson) also published his findings just months ahead of Stevens. Wilson was a good friend of Thomas Hunt Morgan’s and he was aware of Nettie’s research, giving her credit in the footnotes of his paper. Yet, he did not agree with her interpretation and was incorrect in his assumption that sex was determined by environmental factors in Hemipteran insects rather than by sex chromosomes. However, it was Wilson who was internationally recognized for the discovery of sex chromosomes, not Stevens. Moreover, just a year after the publications, Morgan and Wilson were invited to speak at a conference to present their research on sex chromosomes whereas Stevens was not invited (Ogilvie and Choquette 1981). Even so, historians recognize that Stevens’ conclusions were more robust and Wilson did not quite understand the implications of the discovery while Stevens understood just how highly consequential they were (Brush 1978; Kingsland 2007). Her foundational research led to numerous discoveries about the genetics of sex.
A brief review of Chromosomes, DNA, and Genes
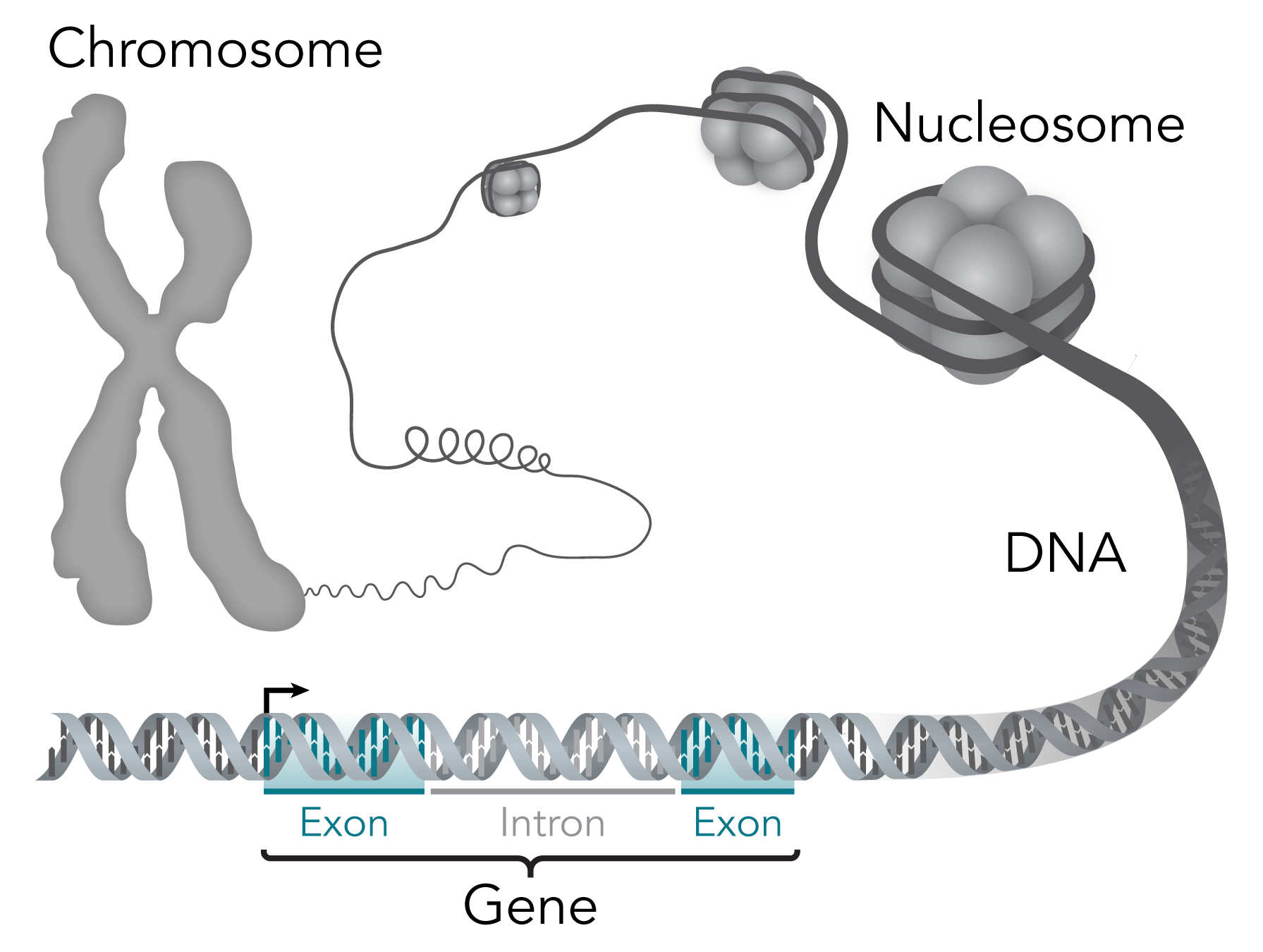
Humans have 23 pairs of chromosomes, twenty-two of which are autosomes that do not differ between the sexes. The remaining two chromosomes, the X chromosome, and the Y chromosome, are sex chromosomes that differ between biological females and males (XX and XY respectively). Chromosomes are only visible during the M phase of the cell cycle (see previous chapter). They are the condensed packaging units of the DNA (deoxyribonucleic acid), a double-stranded molecule that encodes the genetic information in its sequence which is comprised of four nucleic acid monomers guanine, thymine, cytosine, and adenine (G, T, C, & A) pictured on the right. Each chromosome consists of both DNA and associated proteins. The structure of a chromosome is hierarchical and organized at multiple levels, allowing for efficient packaging and proper distribution into daughter cells during the M phase. During most of a cell’s lifetime, a portion of the DNA is in a less condensed state known as chromatin which allows the cell to have access to its information. This is because within the DNA lies an organism’s genes which are specific segments of DNA that contain the instructions for building proteins and RNA (ribonucleic acid) molecules. Each gene is comprised of a series of nucleotides with every three bases encoding information for a particular amino acid (protein monomer) known as a codon.
Codons serve as the basic unit of the genetic code, and they are the “words” that specify the amino acids to be incorporated into a protein during protein synthesis. There are 64 possible codons, which correspond to the 20 standard amino acids and three “stop” codons that signal the end of protein synthesis. During transcription, the gene is read and transcribed into a single-stranded messenger RNA (mRNA) molecule. The mRNA is then translated by ribosomes into a sequence of amino acids which then fold to create a functional protein. Collectively the process of transcription and translation is referred to as gene expression and it is highly regulated. The complete set of an organism’s genetic material is known as its genome however only a small portion of the DNA contains protein-coding genes. When writing about genes and their respective proteins the gene name is usually in lowercase letters and italicized whereas the protein is capitalized, (e.g. sry is the gene and SRY is the protein).
In animals as diverse from one another as fruit flies are to mammals, genes play a central role in the determination of biological sex. However, the location of genes, the number of genes involved, the timing of their expression, and the influence of environmental factors differ and thus a single universal method of sex determination across all species does not exist. Sex determination mechanisms can be quite diverse and can differ even among closely related species.
How is biological sex determined?
Biological sex is not to be confused with gender, they are two different concepts. Biological sex is associated with multiple biological attributes including hormone levels, sex chromosomes, and reproductive anatomy. Gender is limited to socially constructed roles and behaviors. For more information on the relationship between biological sex and gender see the chapter on Gender and Sexuality.
The bipotential gonad

In nearly all sexually reproducing organisms, biological sex is dimorphic (there are only two distinct forms of sex). Individuals have the potential to produce either eggs or sperm and only on very rare occasions make both. When and how sex determination is initiated is highly variable but all animals share a common feature: the reproductive organs involved in gamete production come from primordial germ cells that are biopotential. Biopotential means that these primordial germ cells can differentiate into either female or male reproductive organs (as depicted in the image on the left). A decision is made during a critical window of time in early embryonic development to determine whether or not the reproductive organs form that produce either eggs or sperm. Sex-determining signals that are responsible for this decision have evolved independently multiple times and there is a high degree of variation across taxa as to how sex is determined (Stöck et al. 2021).
What we know about sex determination comes from mutational studies in the following model organisms: the roundworm (Caenorhabditis elegans), the fruit fly (Drosophila melanogaster), the zebrafish (Danio rerio), and the mouse (Mus musculus). Each offers important insight into the molecular mechanisms involved in the commitment to becoming female, male, or hermaphrodite. The commitment part of the process is referred to as determination and the realization of becoming phenotypically female, male, or hermaphrodite is referred to as differentiation.
Genetic Sex Determination (GSD)
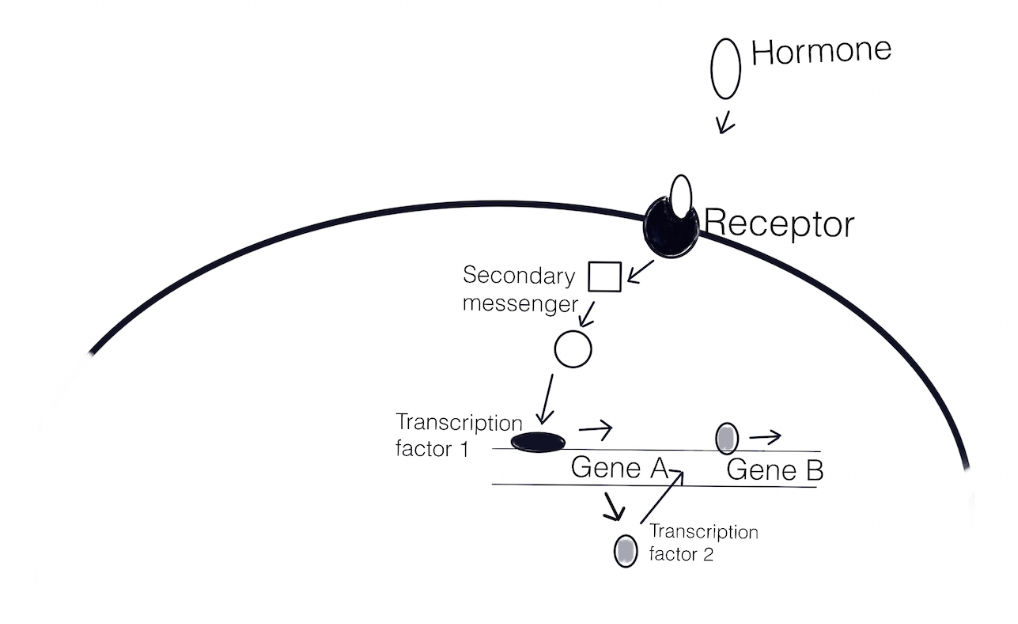
A series of events unfurl after fertilization that orchestrates embryonic development. The overall body plan is determined by the expression of specific genes and the localization of gene products within the embryo. The gene products participate in regulatory pathways (shown in the diagram below) that often work together in a network. A regulatory pathway involves a series of genes being expressed (transcribed and translated) and/or repressed (blocked from being transcribed and translated).
The steps of a regulatory pathway are 1) binding of ligand (e.g. a hormone) to a receptor causes a change that 2) sets off a series of events (secondary messengers convey the signal), and 3) the signal activates transcription factors present to interact with the regulatory elements of genes and express the gene that encodes a transcription factor, 4) the new transcription factors accumulate, and together with other transcription factors, bind to different, previously inactive regulatory elements, and a yet again new state is generated. The simplified illustration below shows how a pathway results in the expression of several genes. This can also be described as a signaling cascade.
To initially kick off the cascade of events, GSD often involves a specific master switch gene “delicately poised to respond to the initial trigger to be male or female and then to amplify this decision” (Swain and Lovell-Badge 1999). As in the case with humans, this switch is localized on the Y chromosome which is responsible for triggering the male-specific cascade. Biological females have a pair of X chromosomes instead and therefore do not have a Y chromosome. These sex-specific chromosomes are referred to as sex chromosomes first discovered by Nettie Maria Stevens in 1905 (see previous chapter), the remaining chromosomes are called autosomes. Suppression of recombination during meiosis led to the differentiation between the sex chromosomes allowing for the evolution of GSD (Charlesworth, Charlesworth, and Marais 2005). The unifying factor in GSD is that the decision lies in the sex chromosomes. Individuals who have two of the same sex chromosomes are referred to as the homogametic sex symbolized as XX (as in female mammals), or ZZ (as in male birds) and those with two differing sex chromosomes are heterogametic as in XY (as in male mammals) or ZW (as in female birds).
Females have two Xs and males one, how is gene imbalance dealt with?
Dosage compensation
In diploid organisms, each cell has two complete sets of chromosomes. However heterogametic individuals have two different sex chromosomes, with one chromosome in the pair significantly larger than the other as in the case of the human X and Y chromosome. This results in unbalanced gene expression because the larger X chromosome has many more genes than the smaller Y chromosome (Bridges 1925). On one hand, this difference is essential for the regulation of sex-specific genes but on the other hand gene imbalance is associated with numerous problems such as disorders and cancer. Therefore mechanisms have evolved to compensate for the imbalance, termed dosage compensation, such as increasing the level of expression of genes on the X chromosome in the heterogametic male or lowering the level of expression of genes on the X chromosome in the homogametic female.
In nematodes (C. elegans) it’s the ratio of XSEs:ASEs
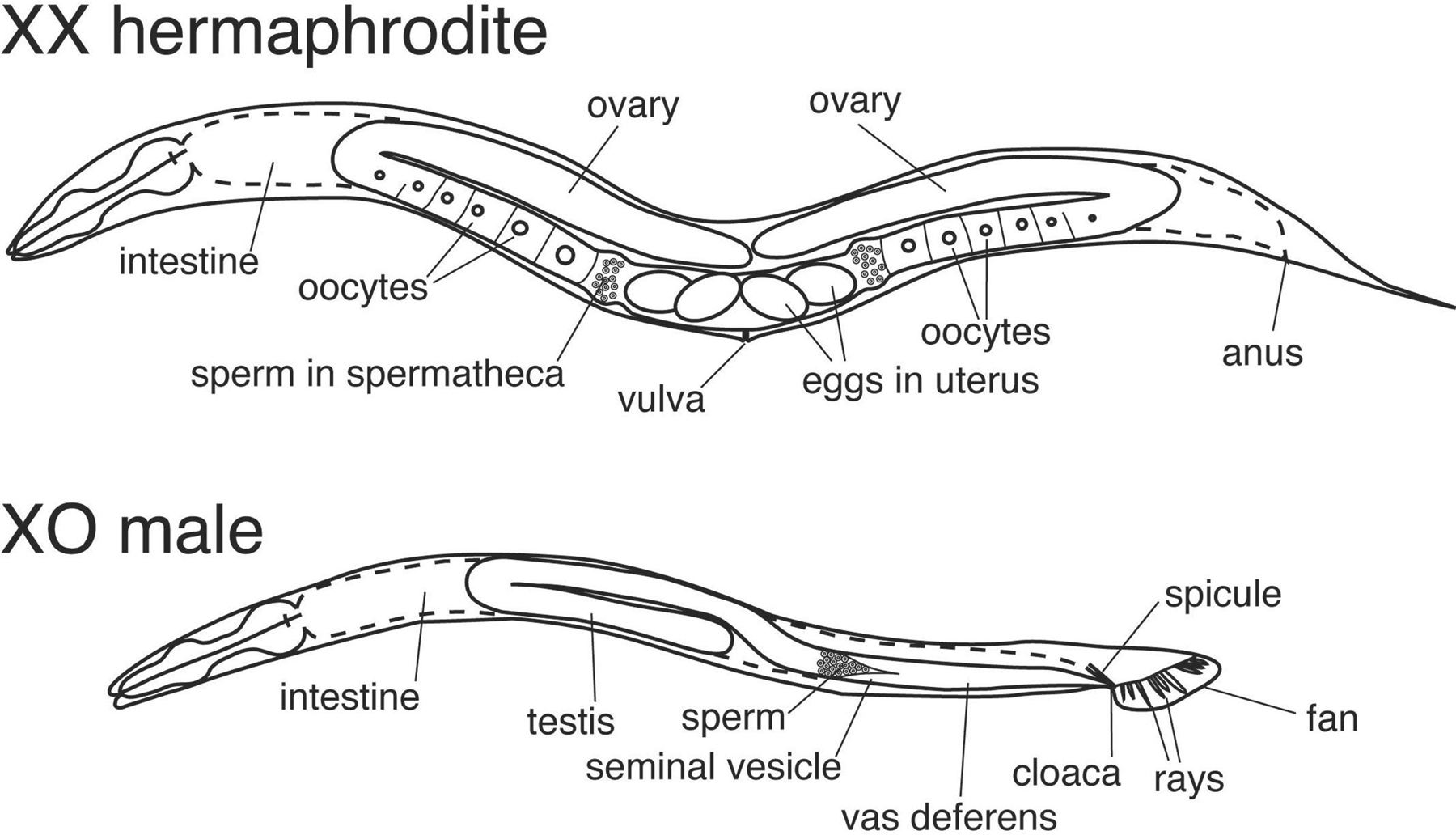
The nematode roundworm, C. elegans, has been a model organism in genetics for some time. They have two different “sexes”: hermaphrodites that undergo both oogenesis and spermatogenesis for self-fertilization pictured below, and males that can produce only sperm to fertilize hermaphrodites via sexual reproduction. Most C. elegans are hermaphroditic and overall their genetic diversity is much lower than that of other obligatory sexual species in the same genus (Félix and Buhay 2010). In the wild, these small roundworms inhabit highly changing environmental conditions that can often lead to long stretches of isolation which may have led to their ability to self-fertilize (Schulenburg and Félix 2017).
The XSEs and ASEs
Whether or not C. elegans becomes hermaphrodite or male is determined during early embryonic development through a series of interacting signals. Barbara Meyer has been investigating the coordinated mechanisms of sex determination and dosage compensation in C. elegans for the past 30 years and provides a thorough review of the topic (Meyer 2022). Meyer notes that it is the ratio of X chromosomes to autosomes (X:A) that is the key signal triggering the “master switch” that determines the cascade of events. The ability to count the number of X chromosomes and Autosomes lies with X-linked genes called X chromosome signaling elements (XSEs) and autosomal signaling elements (ASEs) respectively. These XSEs and ASEs act in opposite ways that result in different transcriptional outcomes. There are several genes involved but the xol-1 gene (XO-lethal) is the master switch, when it is “on” (expressed) the male pathway is chosen, when it is “off” (not expressed) the hermaphrodite pathway is chosen. The pathway is illustrated below.
C. elegans males have a single X chromosome, their genotype is XO because they lack the Y chromosome. This creates an X:A ratio of 1:2, or 0.5, therefore, the ASEs (genes sea-1 and sea-2) have a greater dosage relative to XSEs (genes sex-1 and ceh-39) and they activate the expression of the xol-1 gene. The XOl-1 protein is the master switch protein that sets off a regulatory pathway by activating the transcription of the gene her-1. HER-1 protein binds to TRA-2 receptor blocking TRA-2 activity thereby resulting in male differentiation. This also blocks dosage compensation from being induced since there is only one X chromosome in the male.
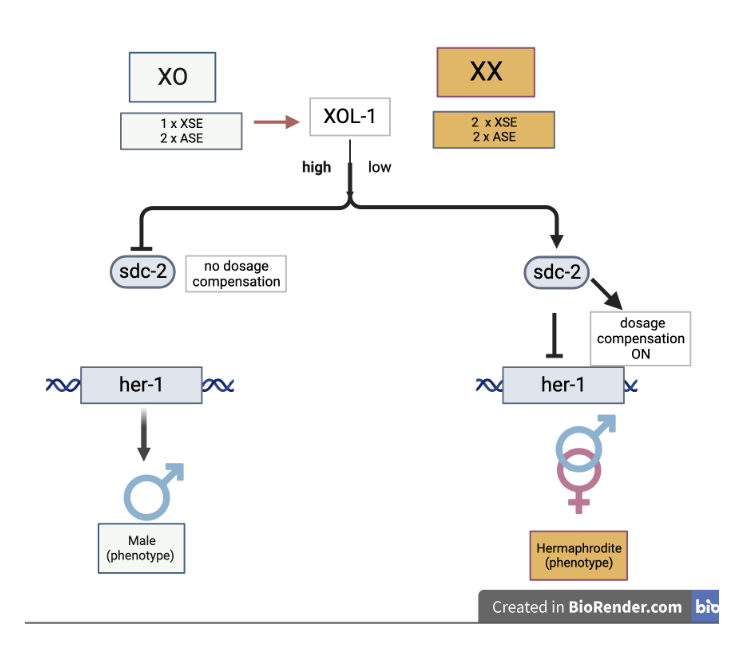
Males only have one X, (XO), the X:A induces high expression of Xol-1, which leads to male-specific gene expression. Hermaphrodites have two Xs (XX), and the greater X:A blocks Xol-1 resulting in female-specific gene expression and dosage compensation.
Hermaphrodite C. elegans results from having two X chromosomes (XX). The X:A is 1:1, equal to one, and this initiates double expression of four XSEs: genes sex-1, fox-1, ceh-39, and sex-2 compared to ASEs. Products of these genes block the expression of xol-1. Without active XOL-1 protein, three genes: sdc-1, sdc-2 and sdc-3 are expressed. The SDC-2 and SDC-3 proteins control dosage compensation by downregulating the expression of the genes on the X chromosomes by half. In this way, dosage compensation is attained. The sdc genes also activate the expression of tra-2 which is needed for expression of the tra-1 gene. Tra-1 proteins are responsible for hermaphrodite development and repression of male development.
In Drosophila, sex is determined by the number of X’s
In Drosophila the master switch gene is located on the X chromosome is called sex-lethal (sxl) gene (Bell et al. 1988), and its female-specific expression is dependent upon the dosage of four additional XSEs (similar to C. elegans). The name sex-lethal comes from mutant analyses: improper expression in males leads to death. Erickson and Quintero (2007) provide a review of the female-specific regulatory pathway. As shown in the diagram below (A) For XX individuals, sxl is “on” resulting in female-specific functional Sxl protein. The functional Sxl protein binds to the pre-mRNA from two other genes: tra (transformer) and tra-2 which encode Tra and Tra-2 proteins. These in turn bind to the dsx (double-sex) pre-mRNA and result in the alternative splicing to make Dsx-F. The dsx gene encodes for a transcriptional regulator, the Dsx-F form that promotes female differentiation and represses male development. SXL is also involved in blocking dosage compensation by binding msl-2 (male-specific lethal) pre-mRNA and promoting splicing that results in a nonfunctional Msl-2 protein. Without the Msl-2 protein interacting with other dosage compensation genes, hypertranscription of genes on the X chromosome will not occur.
The Drosophila sxl gene has two promoters
To ensure the sxl switch stays on in females, the regulation of the sxl gene expression is a bit complicated. The sxl gene has two different promoters, an early female-specific promoter (sxl-Pe), and a maintenance promoter (sxl-Pm). Recall that a promoter is a regulatory sequence just upstream of the start codon in a gene that enables it to be successfully transcribed. Research by Erickson and Quintero (2007) shows that in individuals with two X chromosomes, XSEs are highly expressed in very early development. This is the key to initiating the switch that establishes femaleness. Double expression of XSEs (because there are two X chromosomes) results in the binding of the transcription complex on the sxl-Pe promoter. Illustrated in the diagram below (B), Salz and Erickson (2010) note that Sxl protein expressed from sxl-Pe causes female-specific splicing of Sxl transcripts originating from the sxl-Pm promoter which is activated at a later stage in development. This splicing prevents the inclusion of the poison exon (codon 3, which has a stop codon) in its own primary transcript. The resulting active SXL protein promotes female-specific splicing of sxl-Pm transcripts, establishing an autoregulatory feedback mechanism to keep sxl “on”. Once established, the early promoter is shut off and expression is initiated from the maintenance promoter. How this happens is not clear but the SXL protein keeps sxl gene expression going in each cell throughout the life of the fly (Salz and Erickson 2010).
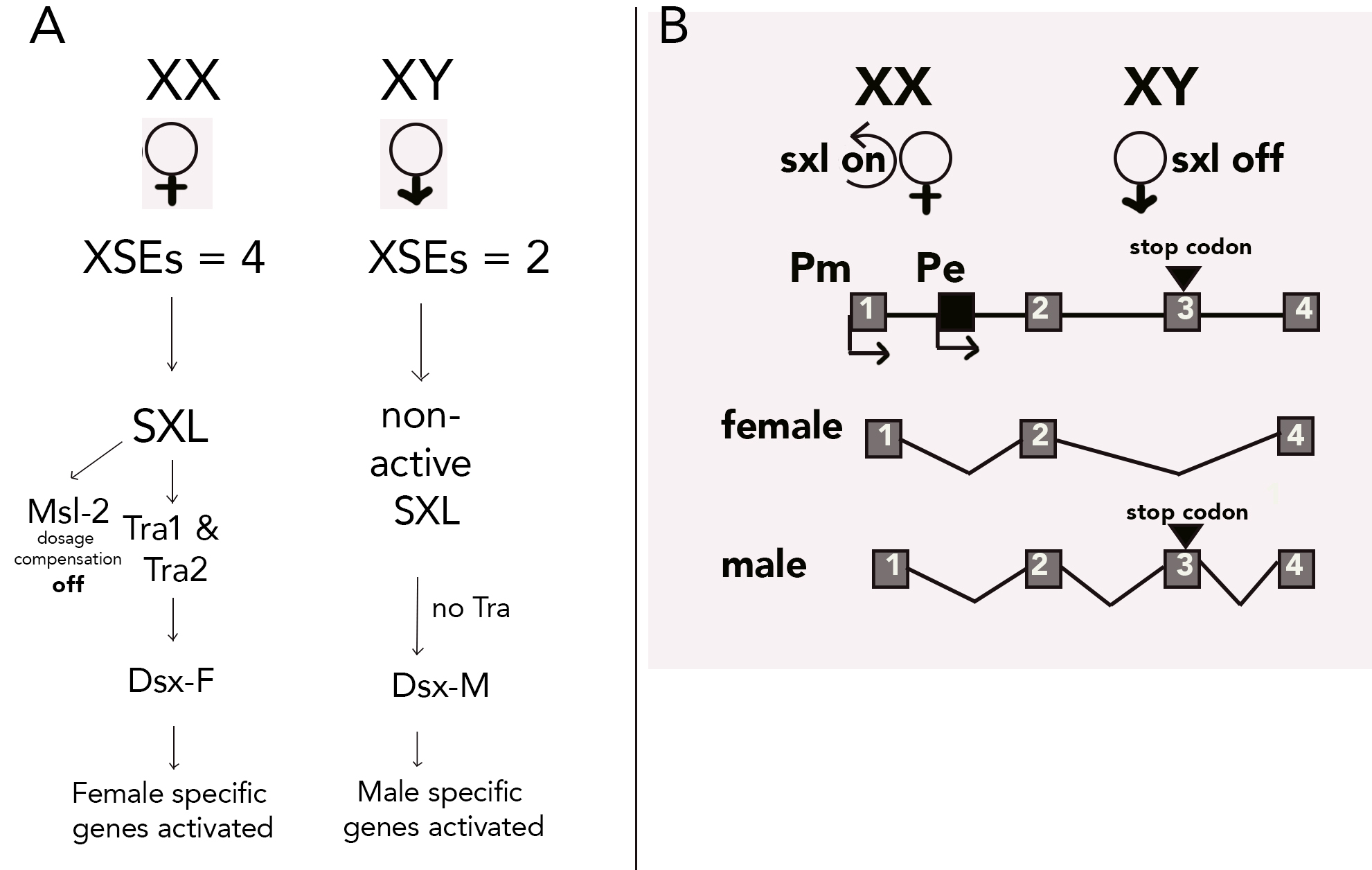
The male-specific pathway happens in flies with only a single X chromosome. The Y chromosome is not involved in sex determination, only fertility. The sxl gene is not expressed from the sxl-Pe promoter, and the autoregulatory feedback loop is off. Instead, transcription happens only from the sxl-Pm promoter but the transcript is spliced in a male-specific way, leaving codon 3 in the transcript, resulting in an inactive SXL (Harrison 2007). Consequently, active Tra is not produced and without active Tra, the Dsx-M form is created. Dsx-M promotes male differentiation. Also, the lack of SXL results in functional Msl-2 protein which promotes overexpression of X-linked genes for dosage compensation (since XY individuals only have a single X chromosome) (Bashaw and Baker 1995).
How is the transcription from the sxl-Pe prevented in males? Early sxl gene expression is blocked by ssx (Sister of Sex-lethal) gene expression whose product is a competitive inhibitor of the sxl auto-regulatory loop (Moschall et al., 2019). Without active SXL protein to bind at the sxl-Pe, only sxl-Pm is bound and this produces an mRNA that includes the male exon with the stop codon, and no active SXL is made. Compared to other insects what happens in Drosophila with sxl as the “master switch” is the exception rather than the rule (Evans and Cline 2013).
Mouse: a model for human sex determination
The mammalian sex-determination system is a XX/XY system. The specific details of molecular mechanisms remain poorly understood due to the complex nature of developing mammalian gonads (Stévant and Nef 2019). Unlike C. elegans or Drosophila, sex determination in mammals is not based on the ratio of XSEs to ASEs, nor the number of X chromosomes rather it is the presence/absence of the master switch gene that determines the fate. The Y chromosome contains the switch: the sry gene (sex determining region of the Y chromosome).
Mammals with two X chromosomes become biologically female
What we know so far comes from extensive studies using mice illustrating that numerous factors are needed to work together in a chorus-like fashion to activate one pathway while simultaneously repressing the other. The review by Eggers, Ohnesorg, and Sinclair (2014) paints a complicated picture of mouse sex determination. During fetal development (10.5 days post-conception), determination is initiated solely in the fetal germ cells located in the genital ridges (gonadal primordia). The sry gene encodes a transcription factor known as testes determining factor (Tdf) and sry expression is activated by several transcription factors including Six1, WT1, and Gata4. The regulatory pathway is depicted in the simplified diagram below. The master gene sry, when expressed produces the protein Tdf that activates the expression of the sox9 gene. The sox9 gene also encodes a transcription factor that leads to testes development by activating the expression of numerous downstream targets involved in Sertoli cell (site for sperm cell production) and Lydig cell (site for androgen production) differentiation such as dmrt1.
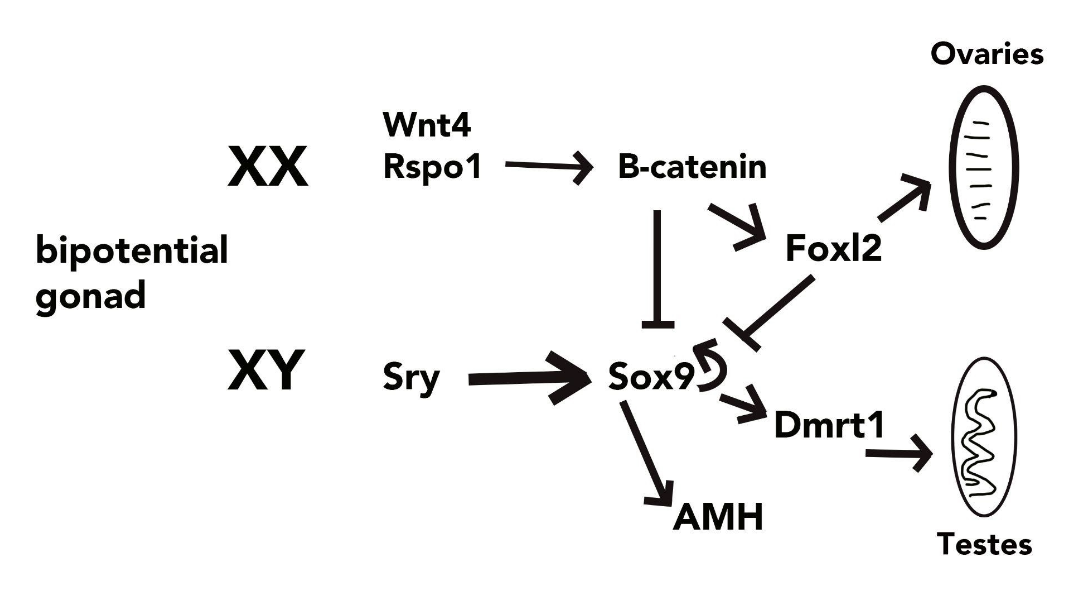
The mammalian female pathway
The female pathway is still not yet well understood. So far several genes involved in the regulation of ovary differentiation have been identified including wnt4, rspo1, ctnnb1, and foxl2.The wnt4 and rspo1 gene products interact to activate the expression of ctnnb1 that produces β-catenin that promotes ovarian development and represses testis-formation. It appears that a balancing act between rspo1 and sox9 is achieved (Rotgers, Jørgensen, and Yao 2018). The foxl2 gene also encodes a transcriptional regulator protein (FOXL2) that suppresses sox9 expression thereby blocking testes formation and this continues throughout adulthood. FOXL2 protein is also important for ovarian cell differentiation (Uhlenhaut and Treier 2006). The dax1 gene product has been shown to block male development but it is also needed for testes development in males therefore the timing of its expression is likely critical to the outcome. Further research is required to elucidate the complexity of the female pathway.
In female mammals, one X is permanently inactivated
Dosage compensation in mammals involves the random shutdown of one of the two X chromosomes, known as X inactivation. Mary Lyon was the first to explain the mechanism, stating that one of the two X chromosomes in female cells is randomly shut down during early embryonic development (Lyon 1961). Moreover, she predicted that all of the daughter cells throughout the animal’s life contain the same inactivated X chromosome. Lyon conducted several genetic crosses in mice supporting her hypothesis (Lyon 1962). This was ground-breaking and she was able to unify several lines of previous experimental data to solve the question of how dosage compensation occurs in mammals (Harper, 2011).
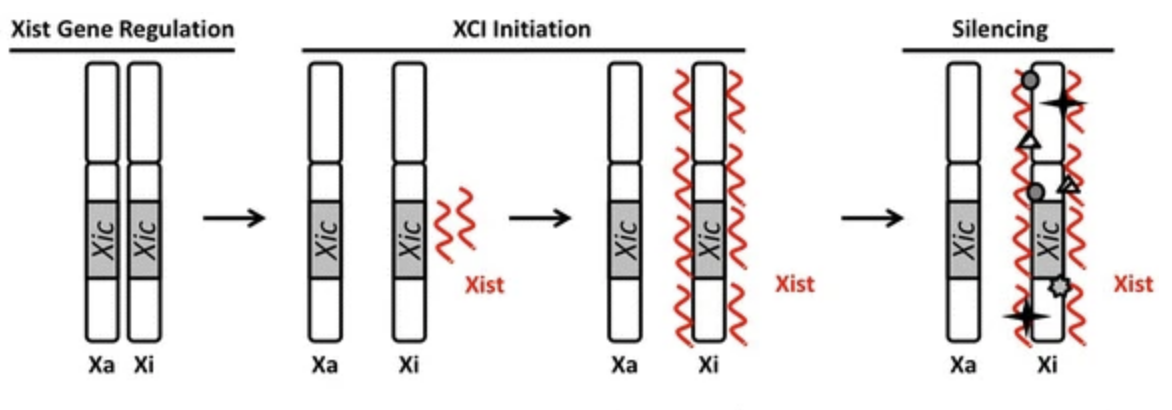
The same X inactivation mechanism occurs in all placental mammals, whereby a long noncoding RNA gene, xist (X inactive-specific transcript) found in a region of the X known as the X-inactivation center (XIC) is expressed from a single X chromosome. The resulting XIST RNA works together with chromatin remodelers to inactivate the X and maintain a silent state (Brown et al. 1992; Plath et al. 2002). Approximately 12-20% of genes located on the X chromosome escape inactivation in humans depending on which tissues they are in, and these genes remain accessible to transcription because they contribute to female-specific differences (Tukiainen et al. 2017). According to Zito et al. (2023) the “genes that escape inactivation have both heritable and environmental components…with genome-wide effects and mutations that impact the escape have complex phenotypical consequences.”
How does XIST work to inactivate an X?
Xist RNA provides a structure for the assembly of other epigenetic factors, changes are modifications to DNA that regulate whether genes are turned on or off, making the Xist RNA essential for X inactivation (Lu et al. 2016). The precise mechanism is still being determined but according to the review by Brockdorff and Turner, (2015) xist is transcribed from one chromosome (Xi), the Xist RNA spreads over and covers the selected chromosome, recruits other proteins that encourage histone modifications and DNA methylation thereby promoting condensation of the chromosome into a Barr body. An antisense Xist transcript is transcribed from the tsix gene on the remaining X chromosome (Xa) to block xist from being expressed or binding to the Xa which remains active (illustrated below). When the cell divides the Barr body is replicated and remains compacted.
The number of X chromosomes in the cell is counted so that only one will remain active. Evidence suggests that autosomal factors stimulate Tsix expression with a competition between Xist repression and activation and the choice is usually random but may be biased for heterozygous alleles. The phenomenon known as skewed X inactivation occurs when “more than 75% of cells in an individual choose the X chromosome from one parent as the Xi” (Sun et al., 2022). A recent study by Dou et al., 2024 suggests in damaged cells Xist RNA and associated proteins act as antigens stimulating autoimmune attack which may be the reason females experience such a high incidence of autoimmune disease compared to males.
Mammals with XY chromosomes become biologically male
To block the female developmental pathway illustrated above, Sox9 activates the amh gene (Anti-Mullerian Hormone, also known as MIS) which causes the regression of the female ducts, and Sox9 is needed for the repression of female-specific genes. Maintenance of the male pathway is assured by continued expression of sox9 (it autoregulates itself) and dmrt1 which both act to keep up testicular development and block the female pathway.
The human X and Y chromosomes
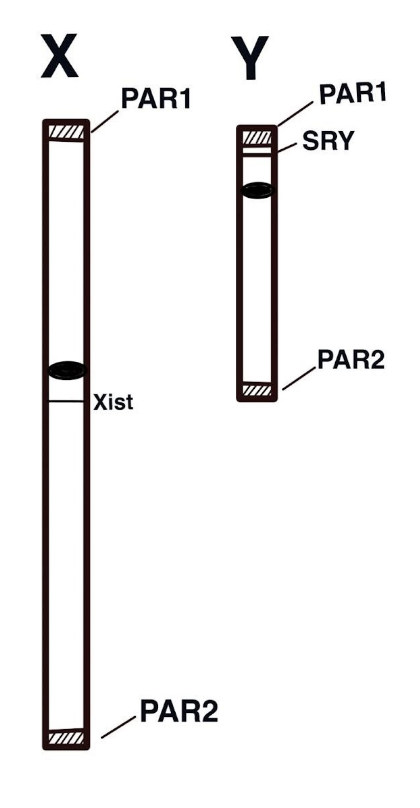
Between 180 to 210 million years ago, the mammalian X and Y chromosomes evolved from autosomes in the placental mammal lineage (Lahn and Page 1999). Recombination is an important feature of prophase I of meiosis yet for most of its length the recombination is suppressed on the Y chromosome. The resulting non-recombining male-specific region (MSY) remains differentiated from the X and is home not only to the sry gene but also to several other genes needed for male fertility. This lack of recombination has allowed the Y chromosome to stay distinct from the X chromosome but it has also led to its degradation (Charlesworth and Charlesworth, 2000). Compared to other chromosomes in the genome, the Y chromosome experiences significantly high rates of mutation from DNA replication error because the male germ line undergoes a greater number of cell divisions than the female germ line (Makova and Li 2002). So the genes located on the X chromosome evolve more slowly in comparison to those located on the Y (Kvikstad and Makova 2013). Until recently, much of the Y chromosome had yet to be sequenced due to its repetitive nature. However, an effort led by the Makova lab at Penn State and Phillippy at the Human Genome Research Institute, successfully filled in the gaps (Rhie et al., 2023). The Y chromosome encodes 66 protein-coding genes, and several genes have a male-specific role.
Both X and Y chromosomes have two pseudoautosomal regions (PAR1 and PAR2) near the telomeres (tips) which are short homologous regions that allow for proper alignment and recombination during meiosis, illustrated on right. The PAR1 region has over 29 genes that do not play sex-specific roles and do not experience the X chromosomal inactivation described above (Colaco and Modi 2018). A third region of homology, the X-transposed region (XTR) is evidently the result of a duplication event following the human-chimp divergence (Ross et al. 2005).
By comparison, the X chromosome has over 150 Mb DNA sequences, over half of which are repetitive. Although the chromosome has significantly fewer genes than most autosomes it still has appreciably more than the Y chromosome, encoding over 1,400 genes (NCBI, 2023).
Why is it called the X chromosome? McKusick (1962) points out that in 1891 while examining chromosomes from the European firebug in mitosis, Henking found a “peculiar chromatin- element and was uncertain of its nature so he labeled it “X”. Thus, the X chromosome…owes its name to the obscurity of its nature at first discovery.”
Q&A with Dr. Kateryna Makova, PhD

Professor of Biology, Verne M. Willaman Chair of Life Sciences, Penn State University
Why do you study molecular evolution? specifically, what drew you to the sex chromosomes and the role of the Y chromosome in evolution? I have always been fascinated by DNA and molecular biology, but I became specifically interested in molecular evolution during my PhD. studies. As a postdoc at the University of Chicago, one of my projects was on male mutation bias – a higher number of mutations originating in the male versus the female germline. I worked with a fragment of DNA that was present on both the Y chromosome and chromosome 3, and I found that it mutates much faster on chromosome Y. It was also much more difficult to study this fragment on the Y chromosome because of its fast evolution (many of the primers I was designing were not working!), so I was wondering what the structure of the Y chromosome is. This was in the early 2000s. I had no idea it would end up being so complex when we deciphered the complete sequence of the human Y in 2023!
What do you enjoy most about your research? The thrill of discovery! I still get that feeling of excitement when I have learned something that nobody had known before and we I will be able to share it with others. I honestly have an adrenaline rush and goosebumps during these moments. They keep me going! The ability to share these moments with my students and postdocs has been and is still extremely rewarding.
What do you find most challenging about your career? Time. There are only 24 hours in a day! There are so many things I would like to investigate, but I cannot investigate them all. Therefore time management is very important and I must prioritize. I have certainly become much better at this after I became a parent.
What advice do you have for current and future women scientists? Be persistent and never give up. It is also important to make sure that you celebrate every success, even a very small one. And remember to enjoy it. As my PhD. advisor taught me, to do science only if one really likes it. If you find yourself not enjoying what you do most of the time – do something else. Life is too short!
Polygenic GSD in the zebrafish

The XX/XY systems are governed by a single master switch that determines sex. It is important to recognize that not all animals have a “master switch” to determine sex, and sex is not static for all adult animals. In a polygenic system, there is no single “master switch” instead multiple loci found on different chromosomes work together to contribute to sex determination as depicted in the diagram to the right (Kosswig 1964). Zebrafish have been studied for over 50 years and yet the details regarding sex determination for this species are still unclear. Wild-caught zebrafish have ZZ/ZW sex chromosomes but they do not differ in size or significant DNA content (Bradley et al. 2011). Genomic investigations suggest the presence of a sex-specific region on chromosome 4 (Anderson et al. 2012) yet several other genes implicated in sex determination are distributed throughout the genome (Orban, Sreenivasan, and Olsson 2009; Liew et al. 2012) and experimental crosses support multiple loci being involved as in a polygenic system.
The following autosomal genes have been identified by Kossack and Draper (2019) as playing an important role in zebrafish sex determination each having orthologous (similar because they are shared through common ancestry) to mammalian sex-determining genes. The dmrt1 gene is involved in male development, similar to mouse Dmrt1. Mutants develop into female fish. Another important orthologue amh (Amh in mammals) is expressed in both male and female zebrafish. In males, it represses female duct development, and in females, it is expressed in the cells of the ovary. Additionally, the orthologue to mammalian Dax1 (NROB1), nr0b1, encodes a gene product that plays a role in the expression of cyp19a1a, a female-specific aromatase-encoding gene. Aromatase is involved in the conversion of androgen to estrogen, a key hormone involved in female-specific gene expression. Two duplicated orthologues of Wnt4 are present in the zebrafish genome and wmt4a mutants are unable to develop functional reproductive ducts. Duplicated Foxl2 orthologues have also been found in the zebrafish genome, foxl2a and foxl2b. These are implicated in maintaining female differentiated fate.
How each of these genes is regulated has yet to be determined and to make the situation more complicated, lab-raised zebrafish do not adhere to a strict GSD mechanism. They lack apparent sex chromosomes (Sola and Gornung 2001). Sex ratios depart from the expected 1:1, female:male in domesticated lines. There is evidence that suggests under certain conditions zebrafish sex determination may be sensitive to environmental influences as well (Abozaid, Wessels, and Hörstgen-Schwark 2012).
Q&A with Dr. Michelle Kossack, PhD.

Postdoctoral Researcher in the Plavicki Laboratory at Brown University
What drew you to studying zebrafish, and specifically sex determination and reproductive biology? I had always loved the ocean, I taught marine science and SCUBA diving as a teenager. After seeing plastic pollution in the ocean and learning about estrogenic plastics I became interested in understanding how plastics could disrupt sex hormone signaling in our environment. My dissertation research in the mechanisms of sex determination in zebrafish was a natural combination of all of my previous passions. Because zebrafish are a common model for human health, and the genetic forces of sex determination were still unknown, this project not only provided a foundation for understanding environmental endocrine disruption but also could inform human reproductive biology.
What have you enjoyed most about your research? That is such a hard question! I think to be able to sustain a career in research you have to enjoy many different parts of it. I love working with fish and using their unique biology to understand human health. I love analyzing data to answer my hypothesis, and I love communicating findings in my writing and public presentations.
What do you find most challenging about your career? One of my weaknesses is patience. I constantly want to know more, and I want to know it now! But, if you have ever worked in research, you know research does not care about your timetable; it moves slowly and methodically and never works the first time around. I find it challenging to slow down and appreciate each small thing, especially when those things aren’t working in your favor.
What advice do you have for current and future women scientists? Define your own success. As you build your career two things will be true: one, there will always be more you can or could do, and two, others will always appear to be achieving more than you. You have to define what you want and work to achieve what you want because it is what you want. In the pursuit of your goal, celebrate early and often! Each step towards your goal is worth a celebration. Some days your celebration will be for an experiment finally working, and some days you will celebrate getting a paper accepted. Each is worth congratulating yourself on taking a step towards your goal.
Environmental Sex Determination

In environmental sex determination (ESD) the key signal(s) that sets off the events culminating in becoming female or male is/are environmental cues to which the developing embryo is exposed during a critical time (Bull 1980). ESD has been observed in all crocodilian species, most turtle species, tuatara, many lizard species, and some fish whereby ambient temperature during incubation is the determining factor (Bull 1983; Mitchell et al. 2006). In crocodilians, research suggests a critical window exists during embryonic development which is sensitive to a narrow temperature range. with most species exhibiting an FMF pattern whereby “low and high temperatures produce a predominance of females, and intermediate temperatures produce mainly males” (González et al., 2019). Although this appears to be quite different from GSD, data indicates a similar underlying mechanism exists involving both commitment to one developmental fate and suppression of the other (Lin et al., 2018).
Why might ESD have evolved?
Phylogenetic studies support the hypothesis that ESD is the ancestral state of sex determination in reptiles, however, it appears to have evolved independently multiple times (Janzen and Paukstis, 1991). Bull (1983) suggests that if environmental conditions are more beneficial to a particular sex, ESD will be favored over GSD because it could produce skewed sex ratios. As in the case with GSD organisms, the reptilian gonad has bipotential primordium that can differentiate into either female or male reproductive organs. The specific molecular mechanisms that occur during determination and differentiation are yet to be discovered but orthologous genes have been found in ESD reptiles that are expressed during embryonic development in a sex-specific manner such as Dax1, Dmrt1, Foxl2, Sox9, and Wt1 (reviewed in (Rhen and Schroeder 2010).
The decision of which path a bipotential gonad will take is important and involves a variety of mechanisms. In the vast majority of organisms, sex chromosomes are where sex-specific genes reside and determine the development of female or male traits.
Those lacking specific sex chromosomes use a polygenic mechanism, environmental sex determination, or a combination to establish the pathway. In all cases, downstream orthologous genes point to a shared ancestry with conserved pathway participants.
Sequential hermaphroditism
For several species of teleost fish, sex is not a fixed fate. Adult individuals change from one sex to another in a process called sequential hermaphroditism (Frisch 2004). These fish can respond to signals often related to body size that stimulate major gonadal and behavioral changes during their reproductive cycle (Wu, Dufour, and Chang 2021). This provides a level of flexibility lacking in those with fixed separate sexes (also known as gonochorism) and may have evolved under situations where the reproductive value of functioning as the opposite sex exceeds that of the current sex. This is known as the sex allocation theory (Warner 1988). Two modes of sequential hermaphroditism have been identified across several different families: (1) protogynous (female-to-male) and (2) protandrous (male-to-female) (Warner 1975). It is yet unclear how sex change in fish is facilitated at the genetic level but as Todd et al. (2016) point out, “sexual fate depends not only on activating one or other sex-specific set of genes but on continued suppression of the opposing network to maintain that fate throughout life…so the sex change likely involves a “manipulation by factors that interrupt supremacy of the prevailing sexual network and allow a takeover by that of the opposite sex.”
Embryonic development of the reproductive system
In mammals, the female reproductive system begins differentiation during early embryonic development. By studying mouse embryo models researchers have been able to deduce the physical changes happening during human embryonic development. A pioneer of mouse embryology was Dr. Ann McLaren (pictured on left) who passed away in 2007. After completing her doctorate at Oxford in 1952, McLaren started studying the role the maternal environment (uterus) plays in embryonic development and she became the first to successfully grow mouse embryos in vitro and subsequently implant embryos into female mice, creating chimeras (McLaren 1976). A chimera contains multiple genetically distinct cell lineages within the same organism. This allowed her to investigate germline mutations and study gene function in the early-developing mouse. McLaren was able to use chimeras to ask key questions about sex determination and germ cell differentiation, furthering an understanding of pluripotent stem cells. A pluripotent stem cell is a type of undifferentiated cell in an early embryo with the remarkable ability to differentiate into various cell types within the body. McLaren published 300 papers and was the first woman to hold office in the Royal Society, she was presented with the title Dame Commander of the British Empire in 1993. Tam and Lovell-Badge (2007) described McLaren’s groundbreaking work as “outstanding” and stated how she had a “positive and enduring influence across many topics in biology.”
Overall, McLaren had a huge impact on our understanding of embryonic differentiation. Differentiation and fetal development in utero is a complex process involving several genes, embryonic cell positioning and communication, and the maternal uterine environment. Below is a general overview of differentiation post-fertilization of the transformation of the zygote from an embryo to a fetus (see illustration below).
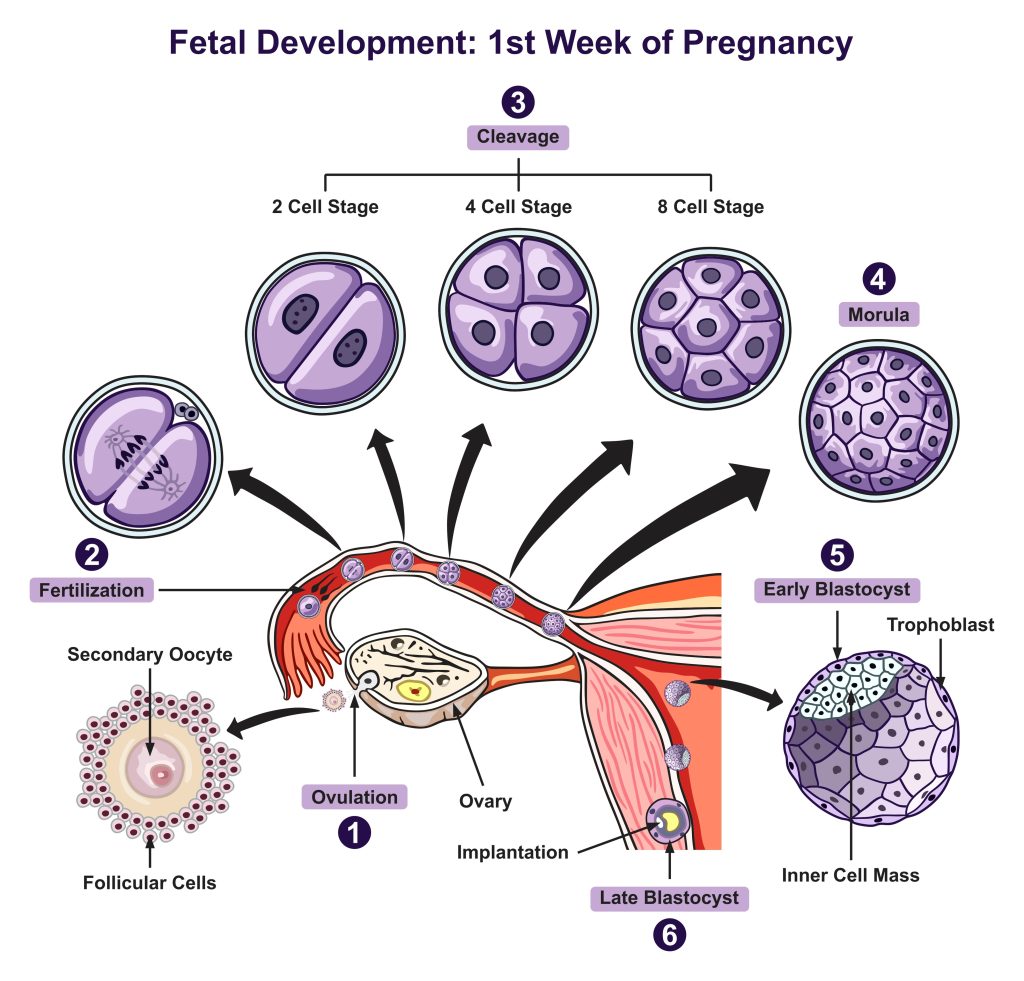
Embryonic development terms
Cleavage: A series of mitotic divisions whereby the volume of egg cytoplasm is divided into numerous smaller nucleated cells called blastomeres.
Morula formation: The cells of the compacted embryo divide and produce a 16-cell small group of internal cells surrounded by a larger group of external cells.
Blastocyst formation: 64-cell stage characterized by an outer layer (trophoplast) and inner cell mass (ICM); the trophoblast gives rise to the placenta and the ICM gives rise to the fetus. The ICM cells are pluripotent stem cells.
Implantation: ~6-7 days post conception (d.p.c.) having traveled down the fallopian tube the blastocyst reaches the uterus and then attaches implants into the uterine lining.
Gastrulation: The cells of the blastula are rearranged into the three germ layers: ectoderm (outer layer), endoderm (inner layer), and mesoderm (interstitial layer).
Germ layer differentiation: Each germ layer gives rise to different tissues and organs in the body; the ectoderm develops into structures such as the nervous system skin and hair, mesoderm gives rise to the muscle-skeletal system circulatory system and reproductive organs, and the endoderm forms the lining of various internal organs.
Organogenesis: Once the three germ layers are established organogenesis begins during this phase the cells within each germ layer undergo further differentiation and specialization to form specific tissues and organs.
Fetal development: As differentiation and organogenesis progress the embryo transitions into a fetus. At this stage, the major organs and systems are formed and throughout the remaining gestational period, the fetus undergoes further growth refinement, and maturation.
Human Embryonic Development Overview
The Bipotential Gonad
In mammals, sex determination begins with the expression of genes on the sex chromosomes (discussed above) leading to differentiation in the gonads. Expression of genes leads to sex steroid hormone production in the gonads that triggers appropriate differentiation of the ducts, glands, and associated genitalia and the decision must be maintained throughout life. The gonad forms within the developing urogenital system from the mesoderm. In the early embryo, all organs normally differentiate into only one type of organ. However, the gonads are exceptional in that regard because they are bipotential (having the ability to differentiate into either an ovary or testes) (for review see Brennan and Capel 2004).
Before the regulatory pathway of determination is triggered to become ovary or testes the embryo is in an indifferent stage that is characterized by rudimentary gonadal ridges formed from primordial germ cells (precursors to eggs and sperm). The genital ridges can be recognized by week 4 of gestation. These gonadal ridges are bilateral, ridge-like protrusions that appear ventromedially to the nephrogenic cord (a transient structure that forms during the early stages of kidney development). During this indifference stage, the epithelium of the genital ridge proliferates into the loose connective mesenchymal tissue above it. Separate from the gonads themselves, a subset of cells in the early embryo is induced to become primordial germ cells. Primordial germ cells migrate to the genital ridge (illustrated below) and become enclosed by supporting cell progeny. These germ cells undergo several successive rounds of mitosis to establish the germ cell pool.
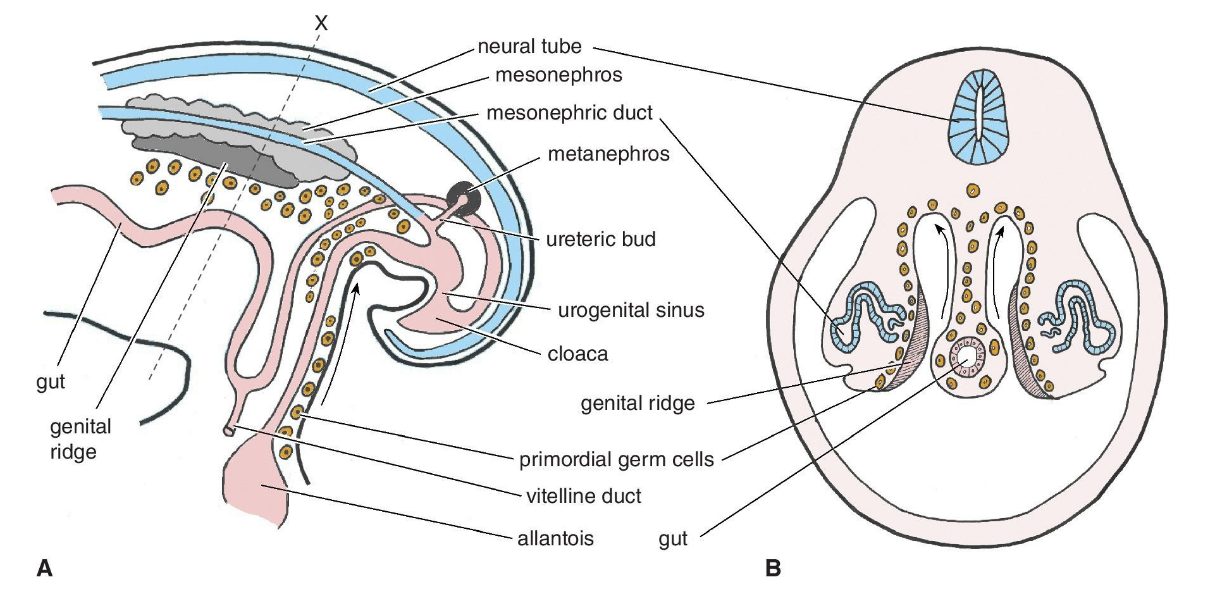
Formation of sex cords
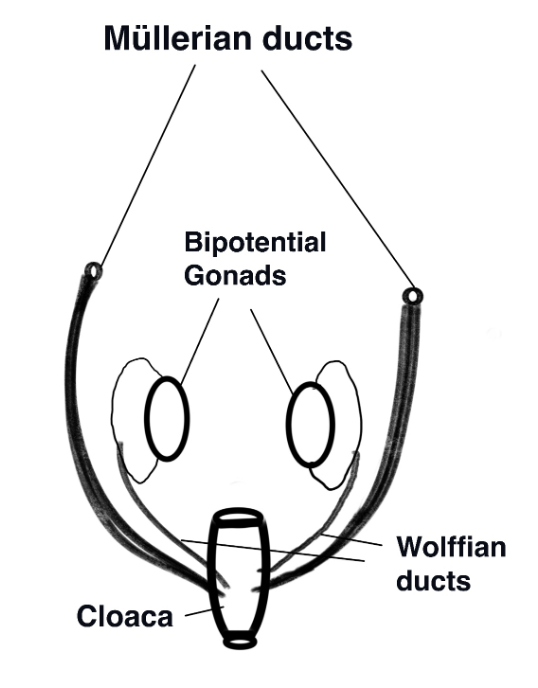
Once the gonadal primordium has been established the gonad is “poised to embark on the journey toward either a testis or an ovary” (Rotgers, Jørgensen, and Yao 2018). The epithelial layers of the genital ridges form the primary sex cords which will surround the primordial germ cells that migrate into the human gonad during week 6. By 7 weeks two sets of internal ducts are present: Müllerian and Wolffian (shown on the diagram to the right). Müllerian ducts are comprised of a meso-epithelial tube surrounded by mesenchyme with an outer layer of epithelium that will differentiate into the female Fallopian tubes (oviduct), uterus, and upper vagina. A comparison of vertebrate taxa from sharks to primates reveals that the initial stages of differentiation of the Müllerian ducts are a highly conserved pathway (Major et al. 2022).
Ovarian and Müllerian differentiation
In the XX fetus at approximately week 8 the sex cords regress and are replaced by a new set of sex cords that remain near the outer surface cortex of the organ (cortical sex cords). The cortical sex cords are divided into clusters called rete ovarii that each surround a germ cell that becomes the ova. The surrounding epithelial sex cords will differentiate into the granulosa cells and the mesenchyme cells of the ovary differentiate into the thecal cells. Granulosa and thecal cells form the follicles that develop into germ cells and secrete steroid hormones. The Müllerian duct remains intact and it differentiates into the paired epithelial tubes (oviducts), uterus, cervix, and upper vagina. The Wolffian ducts degenerate.
External embryonic genitalia
The external genitalia develops in the female as follows: at 8 weeks gestation a genital tubercle, the genital folds, urethral folds, and a urogenital opening are visible. The genital tubercle gives rise to the clitoris and the urethral groove persists as the vestibule. The unfused urethral folds become the labia minora, and the unfused labioscrotal folds become the labia majora.
Male differentiation
In the XY fetus proliferation of the sex cords continues, extending deep into the connective tissue (medullary sex cords). They form a network of internal medullary testis cords and rete testis cords. Apoptosis, or controlled cell death, of the Wolffian ducts is blocked. Instead, the development of the epididymis, vas deferens, seminal vesicle, and efferrent ducts from the Wolffian ducts occurs. The internal cord cells become Leydig cells that make testosterone and Sertoli cells which mature into sperm. Testosterone levels gradually increase as testicular development progresses. Antimüllerian hormone (AMH) is produced by the Sertoli cells, causing the Müllerian duct to atrophy.
The role of sex hormones in embryonic development
Sex determination is made when the genetic switch activates the transcription of downstream target genes. These targets result in the production of steroid hormones: estrogen and testosterone that in turn cue morphogenesis of the ovary or testes. The steroidogenic theca cell and Leyding cell lineages produce these hormones that contribute sexual dimorphism of the embryo. The steroidogenic biochemical pathway (known as steroidogenesis) is complex and involves several types of enzymes to convert cholesterol to steroids. According to a review by Bondesson et al. (2015), two important types of enzymes involved in the pathways are cytochrome P450 (CYPs) and hydroxysteroid dehydrogenase (HSDs). The two classes of sex steroids are estrogens and androgens.
Estrogens
There are three main types of estrogens formed via steroidogenesis: Estrone (E1), 17β-estradiol (E2), and estriol (E3). Cholesterol is converted into androstenedione and then either E1 or E2 is formed. E3 is produced through the conversion of dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) originating from the fetal and maternal adrenal glands. Notably, the placenta keeps high estrogen levels from maternal placental production separate from the embryo.
Estrogens diffuse through membranes and bind to specific nuclear receptors: estrogen receptor alpha ERα and estrogen receptor beta ERβ. When bound to the ER, estrogen-ER moves into the nucleus and binds estrogen response elements on genes to activate transcription. Gene expression microarray analyses of rat XX embryos exposed to synthetic estrogen showed how estrogen was directly involved in the expression of genes involved in tissue remodeling, cell growth differentiation, and apoptosis in the ovary and uterus. Estrogen is produced in XY mammals from circulating androgens (conversion of androstenedione and testosterone) and is required to develop testes (Rochira and Carani 2023).
Androgens
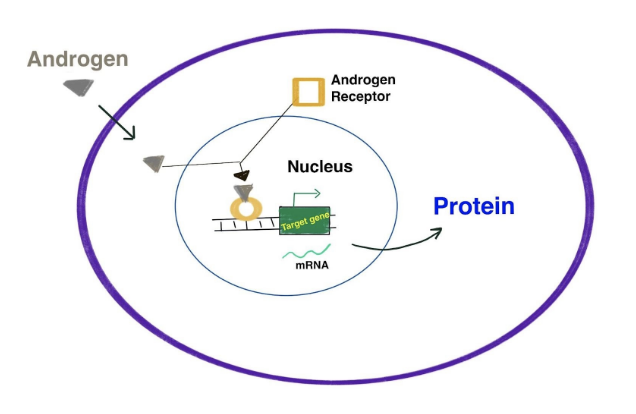
Development of the testes and male duct system derived from the Wollfian ducts requires the action of androgens. Once the testicular cords have been formed Leydig cells start producing androstenedione (~8 weeks), which is converted into testosterone by Sertoli cells. Peak embryonic androgen production is reached around weeks 12-14. Fetal testosterone orchestrates male reproductive development. Similar to estrogens, androgens bind to androgen receptor (AR) and activate the transcription of genes (see image above). Bramble et al. ( 2016) exposed XY mouse embryos to testosterone resulting in gene expression arrays confirming testosterone-induced genes involved in male masculization. The XX fetus is also exposed to low androgen levels produced as a byproduct of corticosteroid production and from maternal adrenal glands (Gitau et al. 2005). These levels are low enough to permit the external genitalia to remain feminine.
Atypical gonadal development
What we know about sex determination and differentiation in humans comes from atypical gonadal development. There is some disagreement on how these atypical instances should be classified (González and Ludwikowski 2016). Traditionally they have been labeled as intersex conditions because the result is often ambiguous genitalia due to a combination of characteristics (eg. XX individual with internal testes). There is a lack of consensus on how intersex should be defined and no simple cause for intersex exists (Davis 2015). More recently the medical community has adopted the classification of disorders of sex development (DSDs) over the term intersex. However, the term “disorder” is also problematic because it pathologizes a person’s condition and has led to misconceptions. There may or may not be a need for someone to seek treatment but the term disorder leads one to think medical corrections should be sought. A more useful way of looking at atypical development would be to take Kessler’s (1998) point of view and think in terms of gonadal variability rather than ambiguity. From a patient advocacy mindset, Davis (2015) reports there is utility in embracing both intersex and DSD, the intersex term can be empowering but the DSD term seems more practical in working with family members and the medical community. Herein we will use the term DSD but it is very important to be mindful and foster understanding, respect, and inclusivity whenever discussing atypical development.
Congenital Adrenal Hyperplasia (CAH)
DSDs are rare, occurring in approximately one in 2000–4500 births (Hughes 2008). The most common 46,XX DSD is Congenital Adrenal Hyperplasia (CAH), (reviewed by El-Maouche, Arlt, and Merke 2017). CAH is due to autosomal recessive mutation(s) in gene(s) involved with enzyme production. This leads to issues in steroidogenesis and can impact glucocorticoid, mineralocorticoid, and sex steroid production. The vast majority involve 21-hydroxylase deficiency (>95%) due to a mutation in the CYP21A2 gene. This can lead to excessive fetal adrenal androgen exposure which results in virilization (development of male characteristics) of external genitalia of 46,XX. However the degree of exposure can vary and patients with non-classic congenital adrenal hyperplasia who are identified by family genetic studies, but asymptomatic are said to have cryptic congenital adrenal hyperplasia.
In 46,XY DSD mutations in DAX-1 have been associated with X-linked adrenal hypoplasia congenita (AHC) which also impacts steroidogenesis, causing an underdevelopment of the adrenal gland leading to impaired pubertal development and male infertility. In very rare cases AHC can occur in girls due to “skewed X-inactivation” (Suntharalingham et al. 2015).
Androgen insensitivity syndrome (AIS)
Hormones must bind receptors and interact with other molecules to be effective. Mutations in androgen receptor (AR) genes are also associated with DSD, known as androgen insensitivity syndrome (AIS) reviewed by Mongan et al.( 2015). The phenotypic effects are dependent on the level of receptor impact and is divided into three categories: complete, partial, and mild form. Complete AIS individuals are 46,XY “with testes which produce age-appropriate androgen levels but have under-masculinized external genitalia due to defects in androgen action” (Mongan et al. 2015). In the partial AIS syndrome, the degree of sexual ambiguity ranges from severe where an XY individual presents with female-like external genitalia to mild with male-appearing genitalia with infertility. Over 400 mutations in AR have been reported thus far.
Mutations in sex-determining pathway genes
Mutations in numerous sex-determining pathway genes have been identified that can lead to partial masculinization of the gonad. For example, 46,XX ovotesticular DSD is a rare subset of DSD characterized by the presence of both testicular and ovarian tissue in the same individual. Mutations in sox3 locus which can induce sox9 lead to testicular development in 46,XX. Additionally, mutations in rspo1, wnt4, and foxl2 also have been identified (Bashamboo and McElreavey 2015). External genitalia exhibit differences in infancy, “however, there are multiple cases of affected individuals who present later in life, as phenotypical males or females” (Kilberg et al. 2019). Mutations in the Y-linked testis-determining gene SRY are responsible for ~15% of XY DSD, and result in partial atypical gonadal development (Xu et al. 2020).
Sex chromosome aneuploidies
Aneuploidy is when there is an abnormal number of chromosomes. Sex chromosome aneuploidies (SCAs), having more than XX or XY may also lead to DSD. Jacobs and Strong (1959) were the first to look at a chromosome spread (known as a karyotype) of an individual who had been diagnosed as having Klinefelter syndrome. This is a diagnosis that was given to males with “small testes and poor facial hair growth” and they found that this individual had an extra X chromosome making them XXY. The common symptom of XXY is infertility and some exhibit cognitive issues due to androgen deficiency. Although there is an increased risk of breast cancer, mediastinal germ cell tumors, and osteoporosis most men live normal lives (Sokol 2012).
Jacobs et al. (1960) describe a XXX individual which they refer to as a “super female”. It turns out that this label is a misnomer since females with an extra X chromosome do not exhibit any apparently unique phenotypic characteristics compared to XX individuals (Hall, Hunt, and Hassold 2006). The impact of extra X chromosomes is likely mitigated thanks to the process of X inactivation.
45,X, Turner syndrome has been associated with short stature, delayed puberty, and infertility. However, those who are mosaic for 45,X exhibit a greater likelihood of menstruation and pregnancy (Zhong and Layman 2012). Turner syndrome mosaicism is a condition that occurs when an individual has a mixture of cells with a normal chromosomal makeup (46,XX or 46,XY) and cells with a single X chromosome (45,X). This is related to the X inactivation pattern of mosaicism that can occur.
Think, Pair, Share
X and Y chromosomes are different in size, the number of genes found on each chromosome, what might be the reasons for this?
Female sex determination in mammals used to be referred to as the “default” pathway. What was the justification for this terminology and is it correct?
In Drosophila dosage compensation involves overexpression of genes on the X in males, but in placental mammals, it is the random inactivation of an X. Why might the mammalian mechanism have been selected?
What role do the sex hormones play in female development?
Deeper Questions
Chromosomal differentiation is key to the evolution of GSD mechanisms, explain how the X and Y chromosomes evolved.
Compare and contrast sex determination mechanisms in Drosophila and C. elegans.
Briefly explain the mammalian female and male determining pathways.
Under what conditions would ESD be selected and what evidence is there that ESD may be ancestral to GSD?
Why might there be redundancies in the developmental pathways for sex?
Why is there disagreement about the term “intersex” vs disorder in describing atypical development?
Key Terms
AIS
Androgen
Androgen receptor
Aneuploidy
Aromatase
Autosome
Autosomal signaling elements
Bipotential gonad
CAH
Determination
Differentiation
Dosage compensation
DSD
ESD
Estrogens
Genital ridge
Genital tubercle
GSD
Heterogametic
Hermaphrodite
Homogametic
Intersex
Lydig cells
Master switch
Mullerian duct
Primordial germ cell
Promoter
Orthologous
Sertoli cells
Sex cords
Sex chromosome
Testosterone
Transcription factor
Virilization
Wollfian duct
X chromosome signaling elements
X inactivation
X inactivation center
Xist
XO
XXX
XXY
References
Abozaid, H., S. Wessels, and G. Hörstgen-Schwark. 2012. “Elevated Temperature Applied during Gonadal Transformation Leads to Male Bias in Zebrafish (Danio Rerio)” Sexual Development 6 (4).
Anderson, J. L., A. R. Marí, I. Braasch, A. Amores, P. Hohenlohe, P. Batzel, and J. H. Postlethwait. 2012. “Multiple Sex-Associated Regions and a Putative Sex Chromosome in Zebrafish Revealed by RAD Mapping and Population Genomics” PloS one 7 (7): e40701. https://doi.org/10.1371/journal.pone.0040701.
Bashamboo, A. and K. McElreavey. 2015. “Human Sex-Determination and Disorders of Sex-Development (DSD).” Plasma Membrane Repair & Development and Pathology of the Gonad in Seminars in cell & developmental biology 45: 77-83. Academic Press. https://doi.org/10.1016/j.semcdb.2015.10.030.
Bashaw, G. J., and B. S. Baker. 1995. “The Msl-2 Dosage Compensation Gene of Drosophila Encodes a Putative DNA-Binding Protein Whose Expression Is Sex Specifically Regulated by Sex-Lethal.” Development (Cambridge, England) 121 (10): 3245–58. https://doi.org/10.1242/dev.121.10.3245.
Bell, L. R., J. I. Horabin, P. Schedl, and T. W. Cline. 1991. “Positive Autoregulation of Sex-Lethal, by Alternative Splicing Maintains the Female Determined State in Drosophila.” Cell 65: 29–239.
Bell, L. R., M. E. Maine, P. Schedl, and T. W. Cline. 1988. “Sex-Lethal, a Drosophila Sex Determination Switch Gene, Exhibits Sex-Specific RNA Splicing and Sequence Similarity to RNA Binding Proteins” Cell 55: 1037–46.
Bondesson, M., R. Hao, C. Lin, C. Williams, and J. Gustafsson. 2015. “Estrogen Receptor Signaling during Vertebrate Development.” Nuclear Receptors in Animal Development 1849 (2): 142–51. https://doi.org/10.1016/j.bbagrm.2014.06.005.
Bradley, K. M., J. P. Breyer, D. B. Melville, K. W. Broman, E. W. Knapik, and J. R. Smith. 2011. “An SNP-Based Linkage Map for Zebrafish Reveals Sex Determination Loci” G3: Genes| Genomes| Genetics 1 (1): 3–0.
Bramble, M.S., L. Roach, A. Lipson, N. Vashist, Eskin, T. Ngun, J. E. Gosschalk, et al. 2016. “Sex-Specific Effects of Testosterone on the Sexually Dimorphic Transcriptome and Epigenome of Embryonic Neural Stem/Progenitor Cells.” Scientific Reports.
Brennan, J. and B.Capel. 2004. “One Tissue, Two Fates: Molecular Genetic Events That Underlie Testis versus Ovary Development.” Nature Reviews. Genetics 5 (7): 509–21. https://doi.org/10.1038/nrg1381.
Bridges, C. B. 1925. “Sex in Relation to Chromosomes and Genes.” The American Naturalist 59 (661): 127–37.
Brockdorff, N., and B. M. Turner. 2015. “Dosage Compensation in Mammals.” Cold Spring Harbor Perspectives in Biology 7(3): a019406.
Brown, C. J., B. D. Hendrich, J. L. Rupert, R. G. Lafreniere, Y. Xing, J. Lawrence, and H. F. Willard. 1992. “The Human XIST Gene: Analysis of a 17 Kb Inactive X-Specific RNA That Contains Conserved Repeats and Is Highly Localized within the Nucleus” Cell 71: 527–42.
Brush, S. G. 1978. “Nettie M. Stevens and the Discovery of Sex Determination by Chromosomes.” Isis.
Bull, J. J. 1980. “Sex Determination in Reptiles.” The Quarterly Review of Biology 55 (1): 3–21. https://doi.org/10.1086/411613.
———. 1983. Evolution of Sex Determining Mechanisms. The Benjamin/Cummings Publishing Company, Inc.
Carey, S. B., L. Aközbek, and A. Harkess. 2022. “The Contributions of Nettie Stevens to the Field of Sex Chromosome Biology.” Philosophical Transactions of the Royal Society B: Biological Sciences 377 (1850): 20210215. https://doi.org/10.1098/rstb.2021.0215.
Charlesworth, D., B. Charlesworth, and G. Marais. 2005. “Steps in the Evolution of Heteromorphic Sex Chromosomes” Heredity 95: 118–28.
Colaco, S., and D.Modi. 2018. “Genetics of the Human Y Chromosome and Its Association with Male Infertility.” Reproductive Biology and Endocrinology 16 (1): 14. https://doi.org/10.1186/s12958-018-0330-5.
Davis, G. 2015. Contesting Intersex. New York University Press.
Dou, D. R., Y. Zhao, J. A. Belk, Y. Zhao, K. M. Casey, D. C. Chen, R. Li, B. Yu, S. Srinivasan, B. T. Abe, K. Kraft, C., et. al. 2024. “Xist ribonucleoprotein promote female sex-biased autoimmunity.” Cell 187(3):733-749. https://doi.org/10.1016/j.cell.2023.12.037.
Eggers, S., T.Ohnesorg, and A.Sinclair. 2014. “Genetic Regulation of Mammalian Gonad Development.” Nature Reviews Endocrinology 10 (11): 673–83. https://doi.org/10.1038/nrendo.2014.163.
El-Maouche, D., W. Arlt, and D. P. Merke. 2017. “Congenital Adrenal Hyperplasia.” The Lancet 390 (10108): 2194–2210. https://doi.org/10.1016/S0140-6736(17)31431-9.
Erickson, J. W., and J. J. Quintero. 2007. “Indirect Effects of Ploidy Suggest X Chromosome Dose, Not the X:A Ratio, Signals Sex in Drosophila.” PLoS Biology 5 (12): e332. https://doi.org/10.1371/journal.pbio.0050332.
Evans, D., and T. W. Cline. 2013. “Drosophila Switch Gene Sex-Lethal Can Bypass Its Switch-Gene Target Transformer to Regulate Aspects of Female Behavior.” Proceedings of the National Academy of Sciences of the United States of America 110 (47): E4474–81.
Félix, M., and C. Buhay. 2010. “The Natural History of Caenorhabditis Elegans.” Current Biology 20 (22): R965–69.
Frisch, A.. 2004. “Sex-Change and Gonadal Steroids in Sequentially-Hermaphroditic Teleost Fish.” Reviews in Fish Biology and Fisheries 14 (4): 481–99. https://doi.org/10.1007/s11160-005-3586-8.
González, E. J., M. Martínez‐López, M. A. Morales‐Garduza, R. García‐Morales, P. Charruau, and J. A. Gallardo‐Cruz. 2019. “The sex‐determination pattern in crocodilians: A systematic review of three decades of research.” Journal of Animal Ecology 88(9): 1417-1427.
González, R., and B. M. Ludwikowski. 2016. “Should CAH in Females Be Classified as DSD?” Frontiers in Pediatrics 4: 48. https://doi.org/10.3389/fped.2016.00048.
Hall, H., P. Hunt, and T. Hassold. 2006. “Meiosis and Sex Chromosome Aneuploidy: How Meiotic Errors Cause Aneuploidy; How Aneuploidy Causes Meiotic Errors.” Genetics of Disease 16 (3): 323–29. https://doi.org/10.1016/j.gde.2006.04.011.
Harper, P. 2011. Mary Lyon and the hypothesis of random X chromosome inactivation. Human Genetics 130(2):169-74. doi: 10.1007/s00439-011-1013-x.
Harrison, D.. 2007. “Sex Determination: Controlling the Master.” Current Biology 17 (9): R328–30.
Hughes, I.A. 2008. “Disorders of Sex Development: A New Definition and Classification.” Fetal and Neonatal Endocrinology 22 (1): 119–34. https://doi.org/10.1016/j.beem.2007.11.001.
Jacobs, P. A., A. G. Baikie, W. M. Court Brown, T. N. Macgregor, N. Maclean, and D. G. Harnden. 1960. “Evidence for the Existence of the Human ‘Super Female.’” Obstetrical & Gynecological Survey 15 (3): 440.
Jacobs, P.A., and J. A. Strong. 1959. “A Case of Human Intersexuality Having a Possible XXY Sex-Determining Mechanism.” Nature 183 (4657): 302–3. https://doi.org/10.1038/183302a0.
Janzen, F. J., and G. L. Paukstis. 1991. “Environmental sex determination in reptiles: ecology, evolution, and experimental design.” The Quarterly Review of Biology 66(2): 149-179.
Kessler, S. 1998. Lessons from the Intersexed. Rutgers University Press.
Kilberg, M. J., M. McLoughlin, L. C. Pyle, and M.G. Vogiatzi. 2019. “Endocrine Management of Ovotesticular DSD, an Index Case and Review of the Literature.” Pediatric Endocrinology Reviews : PER 17 (2): 110–16. https://doi.org/10.17458/per.vol17.2019.kmv.endocrineovotesticulardsd.
Kingsland, S.. 2007. “Maintaining Continuity through a Scientific Revolution: A Rereading of E. B. Wilson and T. H. Morgan on Sex Determination and Mendelism” Isis. 98 (3): 468–88.
Kossack, M. E., and B. W. Draper. 2019. “Genetic Regulation of Sex Determination and Maintenance in Zebrafish (Danio Rerio)” Current topics in developmental biology 134: 119–49. https://doi.org/doi: 10.1016/bs.ctdb.2019.02.004.
Kosswig, C. 1964. “Polygenic Sex Determination” 20: 190–99. Experientia, 20(4), pp.190-199.
Kvikstad, E.M, and K. D. Makova. 2013. “Rapid Evolution of Genes on the Human X Chromosome.” In Encyclopedia of Life Sciences. https://doi.org/10.1002/9780470015902.a0020858.pub2.
Lahn, B. T., and D.C. Page. 1999. “Four Evolutionary Strata on the Human X Chromosome.” Science 286 (5441): 964–67. https://doi.org/10.1126/science.286.5441.964.
Liew, W. C., R. Bartfai, L. Zijie, R. Sreenivasan, K. R. Siegfried, and L. Orban. 2012. “Polygenic Sex Determination System in Zebrafish.” PLOS ONE 7 (4): e34397. https://doi.org/10.1371/journal.pone.0034397.
Lin, J.Q., Q. Zhou, H. Q. Yang, L. M. Fang, K. Y. Tang, L. Sun, Q. H. Wan, and S. G. Fang, 2018. “Molecular mechanism of temperature-dependent sex determination and differentiation in Chinese alligator revealed by developmental transcriptome profiling.” Sci Bull 63(4):209-212.
Lu, Z., Q. C. Zhang, B. Lee, R. A. Flynn, M.A. Smith, J. T. Robinson, C. Davidovich, et al. 2016. “RNA Duplex Map in Living Cells Reveals Higher-Order Transcriptome Structure.” Cell 165 (5): 1267–79. https://doi.org/10.1016/j.cell.2016.04.028.
Lyon, M. 1961. Gene Action in the X-chromosome of the Mouse (Mus musculus L.). Nature 190: 372–373. https://doi.org/10.1038/190372a0
———. 1962. Sex chromatin and gene action in the mammalian X-chromosome. American journal of human genetics 14(2):135-48.
Major, A. T., M. A. Estermann, Z. Y. Roly, and C. A. Smith. 2022. “An Evo-Devo Perspective of the Female Reproductive Tract.” Biology of reproduction 106 (1): 9–23. https://doi.org/10.1093/biolre/ioab166.
Makova, K. D., and W. Li. 2002. “Strong Male-Driven Evolution of DNA Sequences in Humans and Apes.” Nature 416 (6881): 624–26. https://doi.org/10.1038/416624a.
McKusick, V. A., 1962. “On the X chromosome of man.” The Quarterly Review of Biology 37(2):69-175.
McLaren, A. 1976. Mammalian Chimaeras. Vol. 4. CUP Archive.
Meyer, B. 2022. “The X Chromosome in C. Elegans Sex Determination and Dosage Compensation” Current opinion in genetics & development 74: 101912.74. https://doi.org/10.1016/j.gde.2022.101912.
Mitchell, “Support for a rare pattern of temperature-dependent sex determination in archaic reptiles: evidence from two species of tuatara (Sphenodon).” Fronteirs in Zoology 3(9) (2006). https://doi.org/10.1186/1742-9994-3-9
Mongan, N. P., R. Tadokoro-Cuccaro, T. Bunch, and I. A. Hughes. 2015. “Androgen Insensitivity Syndrome.” Nuclear Hormone Receptors for the Clinician 29 (4): 569–80. https://doi.org/10.1016/j.beem.2015.04.005.
Moschall, R., M. Rass, O. Rossbach, G. Lehmann, L. Kullmann, N. Eichner, D. Strauss, G. Meister, S. Schneuwly, M. P. Krahn, and J. Medenbach. 2019. “Drosophila Sister-of-Sex-Lethal Reinforces a Male-Specific Gene Expression Pattern by Controlling Sex-Lethal Alternative Splicing.” Nucleic Acids Research 47 (5): 2276–88. https://doi.org/10.1093/nar/gky1284.
Orban, L., R. Sreenivasan, and P. Olsson. 2009. “Long and Winding Roads: Testis Differentiation in Zebrafish.” Special Issue: Endocrine Models of the Zebrafish 312 (1): 35–41. https://doi.org/10.1016/j.mce.2009.04.014.
Plath, K., S. Mlynarczyk-Evans, D. A. Nusinow, and B. Panning. 2002. “Xist RNA and the Mechanism of X Chromosome Inactivation.” Annual Review of Genetics 36: 233–78. https://doi.org/10.1146/annurev.genet.36.042902.092433.
Gitau, R., D. Adams, N. M. Fisk, and V. Glover. 2005. “Fetal Plasma Testosterone Correlates Positively with Cortisol.” Archives of Disease in Childhood – Fetal and Neonatal Edition 90 (2): F166. https://doi.org/10.1136/adc.2004.049320.
Rhen, T., and A. Schroeder. 2010. “Molecular Mechanisms of Sex Determination in Reptiles.” Sexual Development 4 (1–2): 16–28. https://doi.org/10.1159/000282495.
Rochira, V, and C Carani. 2023. Estrogens, Male Reproduction and Beyond. in Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK278933/.
Ross, M.T., D. V. Grafham, A. J. Coffey, S. Scherer, K. McLay, D. Muzny, M. Platzer, et al. 2005. “The DNA Sequence of the Human X Chromosome.” Nature 434 (7031): 325–37. https://doi.org/10.1038/nature03440.
Rotgers, E., A. Jørgensen, and H. Hung-Chang Yao. 2018. “At the Crossroads of Fate—Somatic Cell Lineage Specification in the Fetal Gonad.” Endocrine Reviews 39 (5): 739–59. https://doi.org/10.1210/er.2018-00010.
Salz, H. K., and J. W. Erickson. 2010. “Sex Determination in Drosophila: The View from the Top.” Fly 4: 60–70.
Schulenburg, H., and M.Félix. 2017. “The Natural Biotic Environment of Caenorhabditis Elegans.” Genetics 206 (1): 55–86. https://doi.org/10.1534/genetics.116.195511.
Sokol, R. Z. 2012. “It’s Not All about the Testes: Medical Issues in Klinefelter Patients.” Fertility and Sterility 98 (2): 261–65. https://doi.org/10.1016/j.fertnstert.2012.05.026.
Sola, L., and E. Gornung. 2001. “Classical and Molecular Cytogenetics of the Zebrafish, Danio Rerio (Cyprinidae, Cypriniformes): An Overview” Genetica 111: 319–412.
Stévant, I., and S. Nef. 2019. “Genetic Control of Gonadal Sex Determination and Development.” Trends in Genetics 35 (5): P346–58. https://doi.org/10.1016/j.tig.2019.02.004.
Stevens, N. M. 1905. Studies in Spermatogenesis. Vol. 36. Carnegie Institution of Washington.
Stöck, M., D. Dedukh, R. Reifová, D. K. Lamatsch, Z. Starostová, and K. Janko. 2021. “Sex Chromosomes in Meiotic, Hemiclonal, Clonal and Polyploid Hybrid Vertebrates: Along the ‘extended Speciation Continuum’.” Philosophical Transactions of the Royal Society B: Biological Sciences 376 (1833): 20200103. https://doi.org/10.1098/rstb.2020.0103.
Sun, Z., J. Fan, and Y. 2022. “X-Chromosome Inactivation and Related Diseases.” Genetics Research (Camb) 2022:1391807. doi: 10.1155/2022/1391807.
Suntharalingham, J. P., F. Buonocore, A. J. Duncan, and J. C. Achermann. 2015. “DAX-1 (NR0B1) and Steroidogenic Factor-1 (SF-1, NR5A1) in Human Disease.” Nuclear Hormone Receptors for the Clinician 29 (4): 607–19. https://doi.org/10.1016/j.beem.2015.07.004.
Swain, A, and R. Lovell-Badge. 1999. “Mammalian Sex Determination: A Molecular Drama” Genes & Development 13: 755–67.
Tam, P., and R. Lovell-Badge. 2007. “Anne McLaren 1927–2007.” Cell 130 (2): 201–3. https://doi.org/10.1016/j.cell.2007.07.016.
Todd, E. V., H. L., S. Muncaster, and N. J. Gemmell. 2016. “Bending Genders: The Biology of Natural Sex Change in Fish.” Sexual Development 10 (5–6): 223–41. https://doi.org/10.1159/000449297.
Tukiainen, T., A.Villani, A. Yen, M. A. Rivas, J. L. Marshall, R. Satija, M. Aguirre, et al. 2017. “Landscape of X Chromosome Inactivation across Human Tissues.” Nature 550 (7675): 244–48. https://doi.org/10.1038/nature24265.
Uhlenhaut, N. H., and M. Treier. 2006. “Foxl2 Function in Ovarian Development.” Molecular Genetics and Metabolism 88 (3): 225–34. https://doi.org/10.1016/j.ymgme.2006.03.005.
Warner, R. 1975. “The Adaptive Significance of Sequential Hermaphroditism in Animals.” The American Naturalist 109: 61–82.
———. 1988. “Sex Change and the Size-Advantage Model.” Trends in Ecology and Evolution 3 (6): 133–36.
Wu, G., S. Dufour, and C. Chang. 2021. “Molecular and Cellular Regulation on Sex Change in Hermaphroditic Fish, with a Special Focus on Protandrous Black Porgy, Acanthopagrus Schlegelii.” Molecular and Cellular Endocrinology 520: 111069. https://doi.org/10.1016/j.mce.2020.111069.
Xu, K., N. Su, H. Zhang, J. Zhu, and X. Cheng. 2020. “A Case Report of 46,XY Partial Gonadal Dysgenesis Caused by a Novel Mutation in the Sex-Determining Region Gene.” Translational Pediatrics 9 (6): 867–72. https://doi.org/10.21037/tp-20-414.
Zhong, Q., and L.C. Layman. 2012. “Genetic Considerations in the Patient with Turner Syndrome—45,X with or without Mosaicism.” Fertility and Sterility 98 (4): 775–79. https://doi.org/10.1016/j.fertnstert.2012.08.021.
Zito, A., A. L. Roberts, A. Visconti, N. Rossi, R. Andres-Ejarque, S. Nardone, J. S. El-Sayed Moustafa, M. Falchi, and K. S. Small. 2023. “Escape from X-Inactivation in Twins Exhibits Intra- and Inter-Individual Variability across Tissues and Is Heritable.” PLoS Genetics 19 (2): e1010556. https://doi.org/10.1371/journal.pgen.1010556.
Suggested Readings
“The Biology of Sex” by Anne Fausto-Sterling – Anne Fausto-Sterling provides a comprehensive overview of the biology and genetics of sex, exploring topics such as sex determination, sexual development, and intersexuality.
“The Evolution of Sex Determination” by Leo W. Beukeboom and Nicolas Perrin – This book delves into the evolution of sex determination mechanisms across different species. It covers a wide range of organisms and examines the genetic and ecological factors influencing the diversity of sex determination systems.
“Sex Itself: The Search for Male and Female in the Human Genome” by Sarah S. Richardson -This book delves into the complexities of sex determination in humans, examining the role of genetics, biology, and culture in shaping our understanding of male and female.
“Temperature-Dependent Sex Determination in Vertebrates” Nicole Valenzuela, Valentine A. Lance (Editors) – This book is a collection of essays that shows how the sex of reptiles and many fish is determined not by the chromosomes they inherit but by the temperature at which incubation takes place.
Interesting Podcasts
From Radiolab: Gonads
