1 Evolution of Sex
STUDENT LEARNING OUTCOMES
At the end of this chapter you should be able to:
- Explain the link between meiosis and sexual reproduction
- Recall how crossing over increases genetic diversity
- Identify the different types of gametes
- Explain why anisogamy is much more common than isogamy
- Illustrate how oogenesis results in a significantly larger gamete
- Recall how anisogamy can be used to define biological sex
- List the costs of sexual reproduction
- Identify the paradox of sex
- Contrast the two main hypotheses for the evolution of sex
Miriam Menkin’s big discovery
Working as a technician on fertility research in Boston at the Free Hospital for Women, headed by clinician John Rock, Menkin (pictured below) made an incredible discovery. Miriam Menkin was the first scientist to successfully fertilize a human egg in vitro (Rock and Menkin 1944). It was the culmination of over six years of working diligently in the lab and although she was the one who did both the intellectual and technical work, as reported in the book by Marsh and Ronner (2019). Her male collaborator, Rock, went on to national prominence while she remained unknown. Forced to relocate to North Carolina when her husband lost his job at Harvard, her research was stopped. Without Miriam in the lab, Rock pivoted from working with IVF and focused his work on the birth control pill. A few years later she returned, however, she was relegated to the position of “literary assistant” editing Rock’s articles (Rodriguez 2015).

Photo of Miriam Menkin with her daughter in the lab, source Harvard University.
Observing the fertilization of a human ovum must have been exhilarating. However, the actual process of fertilization was first studied by Derbès (1847) in sea urchins. Artificially exposing eggs to sperm, Derbès observed that only eggs exposed to sperm could be fertilized. Nearly 30 years later Hertwig (1876) documented the act of fertilization in the sea urchin in great detail, noting that eggs and sperm each have their own nucleus. A year later, Fol (1877) described the penetration of an egg by a sperm whereby the two nuclei fused and formed a zygote. Fol’s observations of fertilization led him to deduce that the substance present in the nucleus, which he named nuclein, our DNA, was reduced in content by half in eggs and sperm and was therefore responsible for the transmission of hereditary characteristics. It took another 67 to confirm this prediction by Avery, Macleod, and McCarty (1944) who published their findings that DNA is the genetic material rather than protein. Just recently AI has been used to better understand how sperm locks onto the surface of an egg during fertilization (Deneke et al., 2024).
In most sexually reproducing species, including humans, females have distinct biological and physiological features and functions that are essential for the process of fertilization, a critical step in sexual reproduction. Let’s examine what sexual reproduction is, how it is related to biological sex, and why it occurs in most multicellular species.
Sexual reproduction vs. asexual reproduction
One of the main characteristics of life is reproduction, the process by which a living organism produces offspring. There are two general ways organisms can reproduce: sexually or asexually.
Defining asexual reproduction
Many single-celled organisms, several plants, and a few multicelled animals can produce offspring without having to find a compatible mate. In asexual reproduction offspring result from the division of duplicated genetic material that produces a clone of a single parent. The offspring are genetically identical to the parent. If there is any variation between parent and offspring it is due to mutation. It is thought that the earliest eukaryotic species were single-cell forms that reproduced asexually through the process of cell division (Wilkins and Holliday 2009).
The specific types of asexual reproduction are apomixis and automixis, for a complete review see (Hojsgaard and Hörandl 2019). Apomixis is common in plants and daughter cells are produced from maternal tissues of the ovule. The daughter cells are produced from a modified cellular division process, without fertilization, and have the same chromosome number and content as the parent cell.
Automixis involves the division of the nucleus that creates sex cells with half the genetic content known as gametes that are used for self-fertilization. The gametes fuse so the offspring are not identical clones. The fusion of two gametes created from the same individual does produce some variation however they are much less genetically diverse than if they were created via sexual reproduction.
Some animals such as whiptail lizards in the genus Aspidoscelis reproduce asexually through parthenogenesis, a type of apomixis in which offspring develop from an unfertilized egg. However, in many cases (as with whiptails) species that reproduce parthenogenetically also engage in sexual behavior (Crews and Fitzgerald 1980).
Defining sexual reproduction
🎵“Let’s talk about sex baby”… 🎵-Salt-N’-Pepa
Most animals reproduce through sexual reproduction. Sexual reproduction involves an individual finding a compatible mate. Together as parents, these individuals each contribute half of their genetic material to produce new individual(s) known as offspring. An important feature of sexual reproduction is that the offspring are genetically different from each parent. To accomplish such a feat specific cells that contain half of the DNA from each parent are needed, called haploid gametes, symbolized as n. The gametes fuse during fertilization to create a full genetic complement, called diploid symbolized as 2n, in the offspring. This is referred to as syngamy which results in a genetically unique zygote the result of the fusion of two different gametes.
Question: Which came first, the chicken or the egg?

The egg came first
As Samual Butler once said: “A hen is only an egg’s way of making another egg.” Individuals produce specialized sex cells each containing half of the individual’s genetic component, known as haploid gametes. In 1885 evolutionary biologist August Weismann studied horse roundworm eggs and concluded that these specialized cells were transmitting a substance that was somehow connected to observable traits in the next generation (Weismann 1885). He postulated the existence of “germ cells”, also known as gametes, contributing half of the genetic material to the next generation. However, it took some time to understand just how gametes were formed through meiosis.
Meiosis produces gametes
Meiosis produces gametes but mitosis was discovered first by Flemming (1882) and we now know that mitosis evolved before meiosis (Wilkins and Holliday, 2009). Mitosis is the mechanism whereby multicellular organisms make more cells for growth and maintenance. The nucleus and cytoplasm of a parent cell divide creating two identical daughter cells, each having the same diploid number (2n) of chromosomes as the original parent cell. The goal of mitosis is straightforward: mitosis properly distributes replicated chromosomes into two genetically identical daughter cells. These new identical daughter cells are necessary for the growth and replacement of damaged cells.
Meiosis is similar to mitosis but occurs only in the germ cells (highly specialized cells that form gametes) and involves two successive divisions (MI and MII) allowing for the reduction division of chromosomal content. The new daughter cells have the haploid (n) number of chromosomes and are gametes. For example, a diploid human somatic cell contains 46 chromosomes but a haploid gamete cell (egg or sperm) contains only 23 chromosomes (n = 23). Below is a diagram comparing daughter cells produced in mitosis versus meiosis.
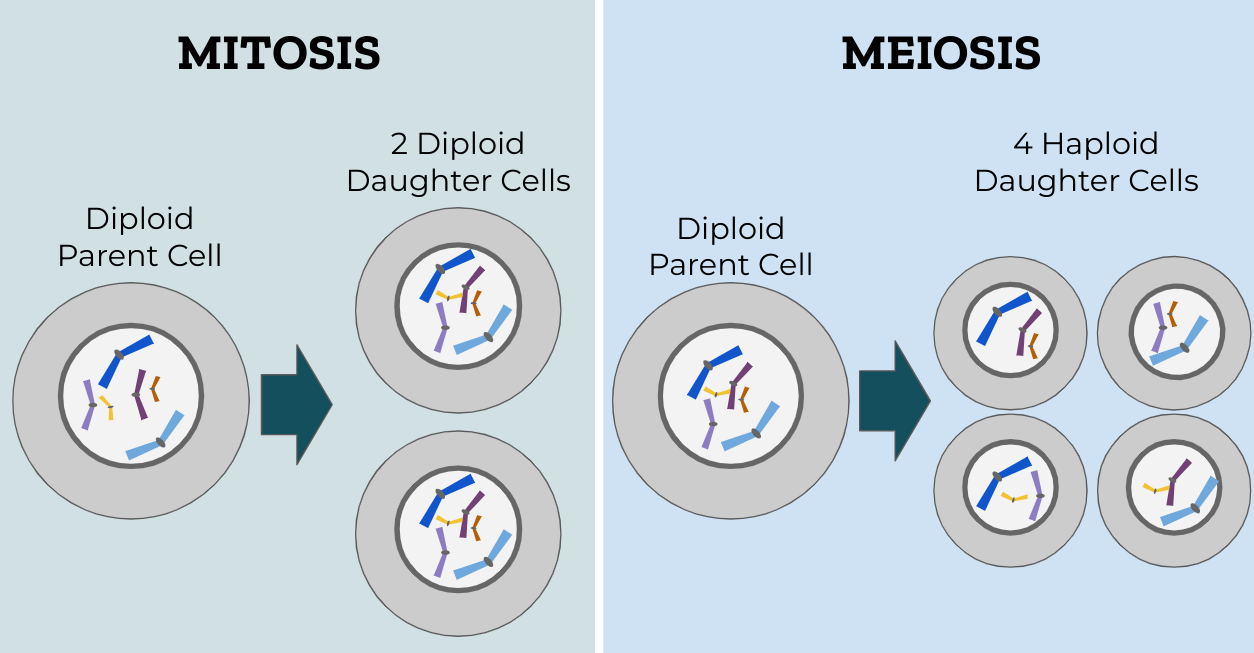
Haploid cells (n) are produced from a cell that was originally diploid (2n). Recall that the cell cycle has four stages, G1, S, G2, and M. During the S phase the DNA is replicated and then packaged into chromosomes at the start of the M phase of the cell cycle. It is during the M phase that nuclear division and cytoplasmic division occur. For a detailed description of meiosis see Meiosis: An overview of key differences from mitosis
Homologous chromosomes pair up and synapse during prophase I of meiosis. Crossing over between non-sister chromatids creates new allelic combinations.
During the M phase of the cell cycle, the chromosomes in the diploid parent cell undergo alignment and division. Initially, two sets of matching chromosomes contain the same genes, but potentially different alleles, which are called homologous chromosomes. During the first stage of meiosis, prophase I, homologous pairs of chromosomes come together allowing for proper alignment of the chromosomes. Proper alignment is necessary for the equal division of the genetic material. This also enables another important event during meiosis known as crossing over. Crossing over (shown in the figure below) is the physical exchange of chromosomal segments between different chromosomes within the homologous pair. This creates new combinations of alleles that contribute to the uniqueness of the zygote. Crossing over, depicted in the diagram below, is also referred to as recombination.
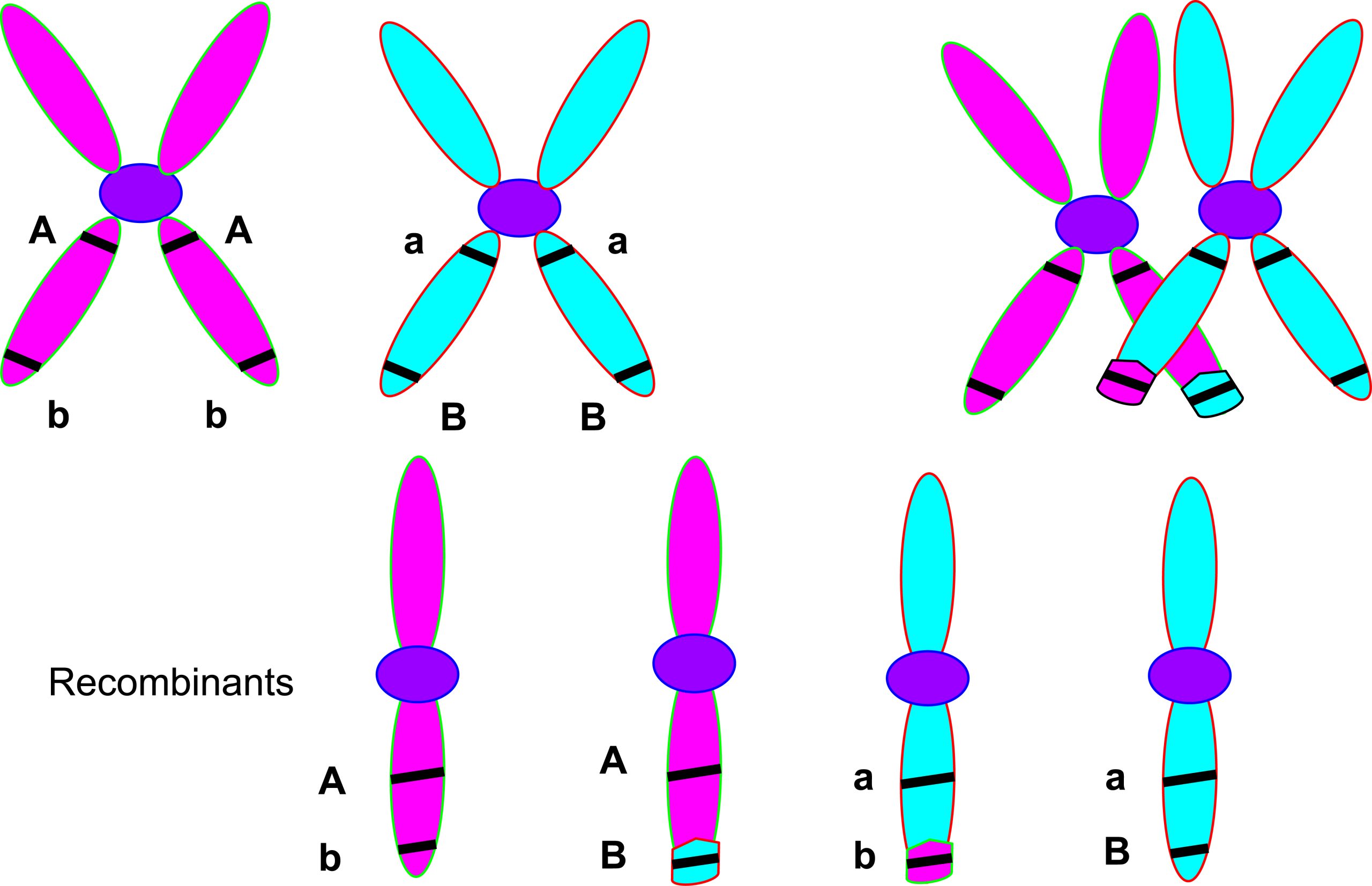
How the chromosomes pair up is random- the maternal chromosome can be on the left of the paternal chromosome or vice versa. With many pairs of chromosomes the random alignment of each results in the independent assortment of genes on different chromosomes. The first physical evidence of different chromosomes assorting independently during meiosis was provided by female geneticist Estrella Eleanor Carothers who examined grasshopper chromosomes during meiosis (Carothers 1917). Unlike mitosis, which only has one division, meiosis has two nuclear divisions (MI and MII) producing four daughter cells. These four haploid daughter cells are genetically unique from each other because they contain only one homologous chromosome from each pair that has undergone crossing over. This is observed among algae in the genus Chlamydomonas . These single-celled organisms undergo meiosis and produce distinct gametes that are genetically unique with different chromosome types. When they mate, one contributes a + type, and the other a – type. These fuse and create a diploid product (+/-) that differs from each parent.
There are a variety of sexual life cycles in eukaryotes, some are haplotonic (the haploid cell undergoes mitosis, eg. algae); diplotonic, (the diploid phase undergoes mitosis, eg. most multi-cellular animals and seed plants; and diplohaplotonic (both undergo mitosis, eg. alternation in generations in some plants). The more complex the organism, the more likely the diplotonic cycle is the case (see review by Heesch et al., 2021).
Meiosis and the transmission of hereditary traits
Gregor Mendel (1866) presented his results in the paper “Experiments on plant hybridization” before the discovery of either meiosis or chromosomes. However, he was able to use experimental crosses of pea plants to deduce each parent has two “factors”, also known as alleles that make up their genotype for each trait. Each of the two parents randomly contributes one of those alleles to their offspring in the next generation. Mendel meticulously crossed seven different sets of pure-breeding pea plants for a single trait or phenotype (each exhibiting two distinct forms, e.g. plants with green pods x plants with yellow pods) which produced offspring that only had green pods. Because all the offspring were green he considered the green pod the “dominant” phenotype. Mendel then self-crossed the green pod first-generation offspring to produce a second generation. This resulted in a consistent pattern of ¾ exhibiting the “dominant” (green) phenotype to ¼ exhibiting the “recessive” (yellow) phenotype.
- Parent generation (P): Green pod x Yellow pod
- First generation (F1): All Green pod
- Second generation (F2): ¾ Green pod; ¼ Yellow pod
This pattern was consistent for each of the seven traits in Mendel’s crosses. Thanks to the large sample size produced from each of the crosses, Mendel was able to mathematically explain the results, concluding that when the parent plants were crossed this was a monohybrid cross, they each had different alleles, represented by letters (e.g. GG and gg) that they passed to the F1 plants (Gg). The F1 plants produced egg and sperm cells that exhibit the “law of equal segregation”. Equal segregation means that each parent has two alleles per trait (Gg) but they only pass on one allele per trait (G or g), and they have an equal chance of passing along either one to the next generation. This is possible thanks to meiosis. Each parent’s diploid chromosomes divide and segregate equally into their haploid gametes.
When Mendel considered two traits together in a dihybrid cross, e.g. green pod and tall plant, (genotype GGTT ) by a yellow pod, short plant. (genotype ggtt), he demonstrated that the factors for different traits are not connected and are randomly passed onto the next generation. Once again his results were consistent for all seven traits in dihybrid crosses. He called this the “Law of Independent Assortment” and was correct because fortunately each of the alleles for these traits is located on different chromosomes. The 9:3:3:1 phenotypic ratio observed in the second generation is a product of two different alleles on different chromosomes aligning randomly during meiosis. The Punnent square below illustrates how the second-generation phenotypic ratio is obtained.
- P: Tall plant with green pods x Short plant with yellow pods
- F1: All Tall plants with green pods
- F2: 9/16 Tall, Green; 3/16 Short, Green; 3/16 Tall,Yellow; 1/16 Short, Yellow
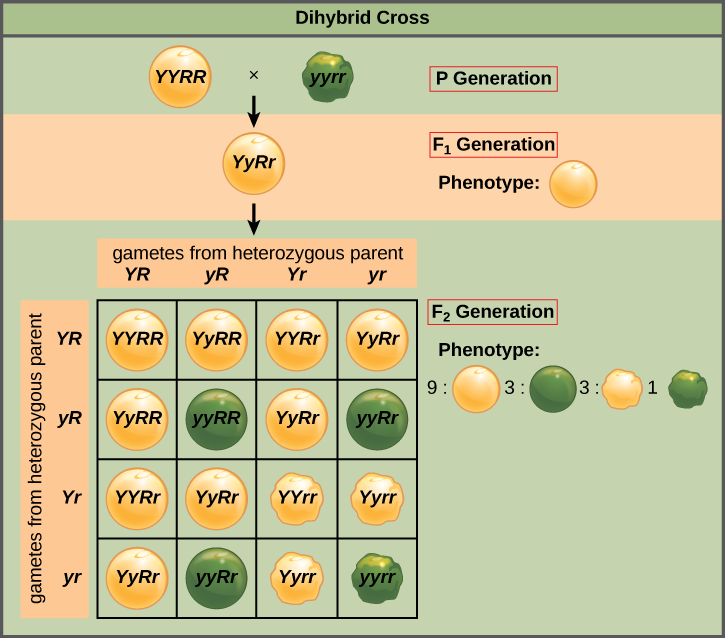
At the time Mendel’s research was published it went unnoticed however it was rediscovered 35 years later by William Bateson (1901) who went on to coin the term “genetics”. Soon after a direct link between cytological evidence (actual chromosomes viewed under the microscope) and Mendel’s principles of equal segregation and independent assortment was made. Working with grasshopper chromosomes, Sutton (1902) established the fact that chromosomes are organized into homologous pairs dividing equally in the first phase of division and then reducing the chromosome number in the second phase of division. He went on to put forth the chromosome theory of inheritance (Sutton 1903).
Theodor Boveri (1904) observed chromosomes actually dividing when gametes were made in roundworms (Ascaris), further supporting the conclusion that the chromosomes were the location of Mendel’s hereditary factors. The confirmation of the chromosomal basis of inheritance was provided by Thomas Hunt Morgan (1911) and his student Alfred Sturtevant (1913) in genetic experiments with the common fruit fly (Drosophila melanogaster). Together they were able to show that genes are located linearly on chromosomes and a locus represents a specific position or site on a chromosome where a particular gene is located. Later researchers were able to demonstrate that genes consisted of DNA. A brief overview of DNA and genes is provided in the following chapter, the Genetics of Sex.
Why are there only two types of gametes (eggs & sperm)?
A brief review of the theory of natural selection
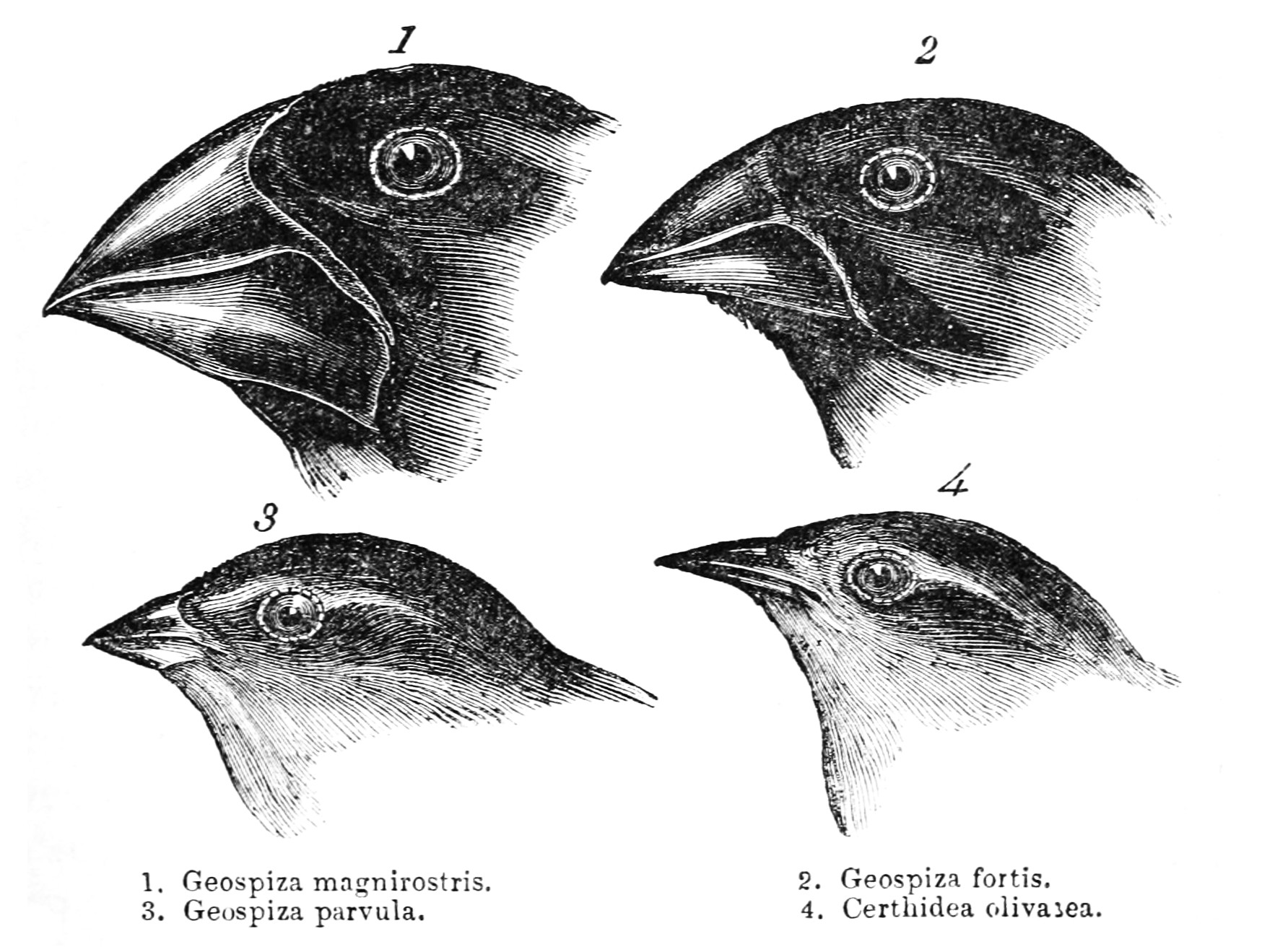
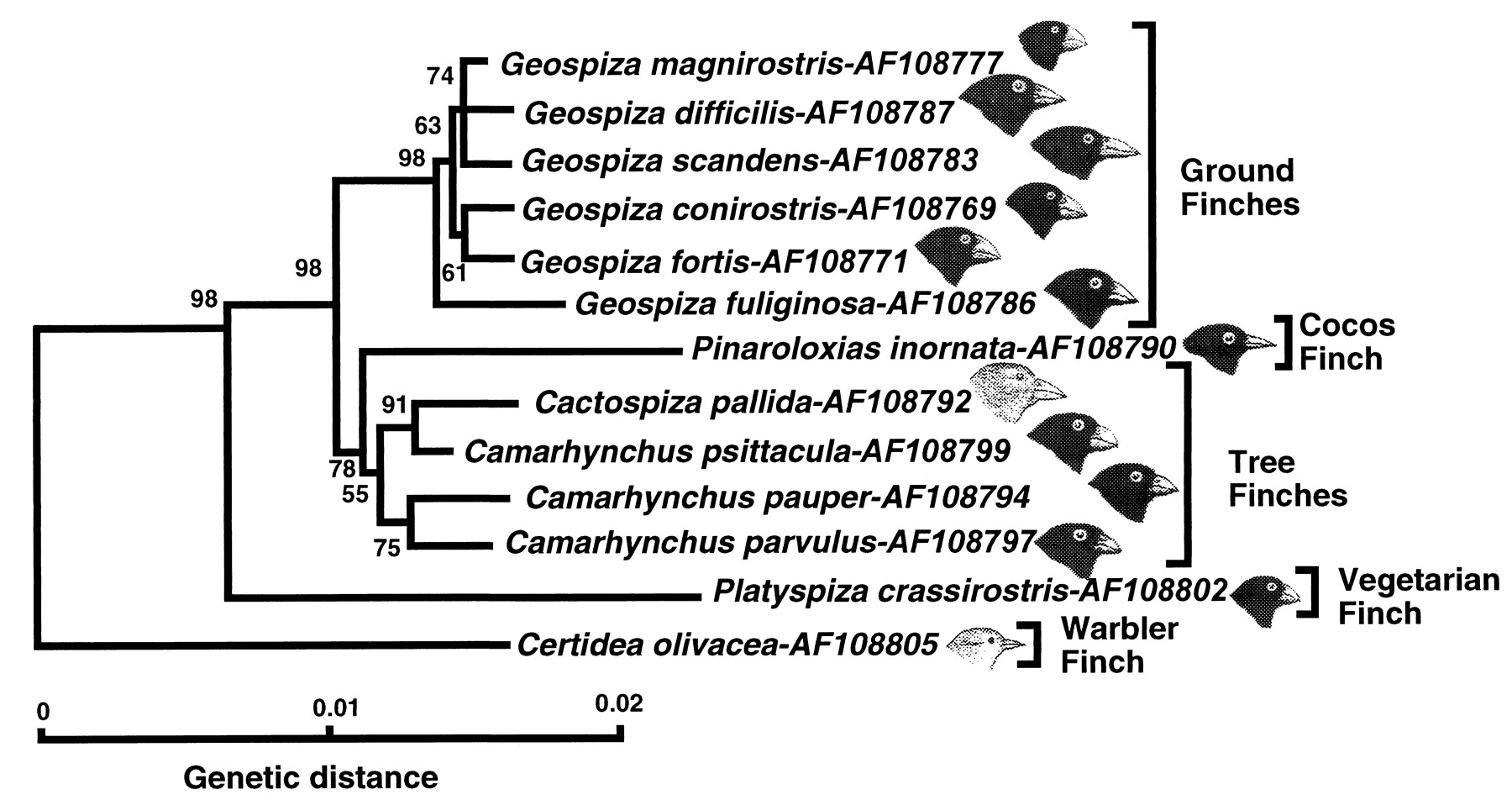
Before we can understand why sexual reproduction is much more common than asexual reproduction, or why there are only two different types of gametes as opposed to several different types we must refresh our memory of evolution and natural selection. Charles Darwin reluctantly published “On the Origin of Species” in 1859 because he was so concerned about the cultural impact it would have on society. In this seminal text, Darwin provided a thorough explanation of evolution and a detailed explanation of his theory of natural selection. This was the first evidence-backed explanation of how populations evolve and new species can arise. The image below is of different finches from the islands of the Galapagos archipelago comes from Darwin’s own field notes that resulted from his voyage around the world. Darwin observed variation within populations and he postulated that if such variations are inherited, then they will be “naturally selected”.
The theory of natural selection can be condensed into four postulates
- Variation exists within populations.
- The heritable portion of this variation is passed to the offspring.
- More offspring are produced than can survive and there is competition for limited resources.
- Those who have inherited traits that increase their reproductive success (fitness) will produce more offspring in the next generation, and their heritable traits will increase in frequency over time.

Evolutionary fitness is directly connected to reproduction. Fitness refers to the number of offspring produced by an individual in the next generation and traits such as survival and reproductive success combine to determine fitness. Natural selection for one trait over another occurs “when there is a consistent, average difference in fitness” (Futuyma 2009) thus selection is the evolutionary force that predominately results in adaptation. Adaptations are traits that increase an organism’s chance of survival. An example of an adaptation is the cryptic coloration found in Botsford’s Leaf-litter toad (Leptolalax botsfordi) pictured on the right, which improves its ability to evade predation in the montane rainforest of northern Vietnam, thereby increasing fitness.
Darwin observed that different species of finches in the Galapagos islands had different shapes and sizes of beaks which he proposed evolved through natural selection. Examination of the phylogenetic tree (shown below) created from the analysis of DNA sequences provides strong support that Darwin’s finches are closely related having shared a common recent ancestor (Sato et al. 1999). However, they have diverged due to ecological differences in their environments that impact food availability. For example, the ground finches have larger beaks for breaking seeds and smaller beaks more suited for capturing insects whereas the tree finches have large beaks used for “tearing bark and crushing twigs and small branches…Darwin’s finches vary in beak shape/size and are versatile in their feeding habits, see illustration below. This versatility is fostered by ecological opportunity…”(Grant and Grant 2003). This is an example of how adaptations in beak shape increase fitness under particular environmental conditions and are thereby naturally selected over other types.
Selection can be subdivided into different categories based upon outcomes: a) directional selection is when one phenotypic trait is consistently favored in a population over time; b) stabilizing selection is when the intermediate phenotypic trait is consistently favored within a population over either extreme; and c) disruptive selection is when both phenotypic extremes are consistently favored over intermediates.
Darwin proposed that natural selection is the chief cause of evolutionary change. However, selection is not the only evolutionary force, mutation, genetic drift, and gene flow also cause changes in populations over time. Keep evolution in mind because it is responsible for many of the features we will discuss throughout this text.
For most species, eggs and sperm are different in size and shape
Although most eukaryotic organisms have only two sexes, sexual reproduction does not require only two different sexes. There exist many unicellular eukaryotes, such as protists and fungi, which exhibit isogamy. Isogamous species produce gametes that are the same size and shape. They are physically indistinguishable but are distinct self-incompatible genetic “mating types”. Often there are two different types designated as + and – like those found in many species of algae such as Chlamydomonas reinhardtii (Ferris and Goodenough 1994). However, Lehtonen et al. (2016) report the number can vary (e.g. three gamete types found in Dictostelium discoideum, seven in Tetrahymena thermophila). These haploid gametes undergo syngamy and produce a viable zygote.
In isogamy the parental investment in the zygote is equal. However, isogamy is found only in single-celled organisms which begs the question why? It may be that larger multicellular organisms require greater nutrient storage (Parker, Baker, and Smith 1972). Hanschen et al. (2018) found that species with large eggs and small sperm (anisogamy) produce larger zygotes than isogamous species, likely leading to anisogamy evolving from isogamous multicellular species multiple times.
Anisogamy is the predominant system for multicellular organisms and is characterized by two different gamete sizes: small, motile (microgametes), and large, immotile (macrogametes). Anisogamy likely evolved from isogamous microgametes (Lehtonen and Parker 2014; Lehtonen, Kokko, and Parker 2016). The increased complexity of multicellular organisms would account for the eventual selection of macrogametes which could be large enough to support a more complex zygote.
Why anisogamy? Larger eggs can supply greater amounts of nutrients and proteins for the developing embryo. So multicellular organisms under natural selection have only two distinct gamete types (dimorphic gametes) that are winners in the game of gamete evolution. The theory for the evolution of gamete dimorphism first posed by Darwin is based on the trade-off between gamete number and offspring fitness. The main functions of a gamete are to find and fuse with another haploid gamete and produce a successful zygote that will go on to survive, mature, and ultimately reproduce. Gamete competition is a selective force resulting in ever-shrinking gametes that increase in number while simultaneously ever-larger gametes providing nutrients.
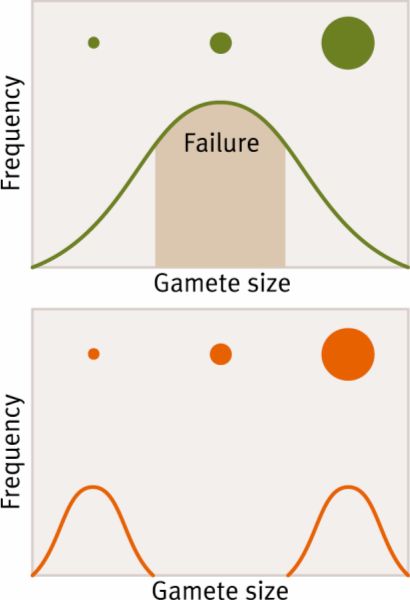
Disruptive selection can explain how two and only two forms are selected (intermediate-sized gametes are selected against) and why smaller gametes favor fusing with larger gametes (Parker, Baker, and Smith 1972). This is illustrated in the diagram to the left. The intermediate size loses because it is neither best at finding other gametes nor best at supporting offspring, one form is favorable for a particular function over another but rarely can you combine different functions into a single form. Therefore gamete dimorphism evolved to increase the likelihood of gametes finding partners which they can successfully fuse (Dusenbery 2002). Small, motile, abundant male gametes increase the chance of finding a partner of the opposite sex when gametes are released into seawater. A larger female gamete encourages a more successful zygote. The microgamete must be able to find the macrogamete, and as larger, allows the macrogamete to emit more pheromones to increase male gamete attraction (Dusenbery and Snell 1995). For a comprehensive review of anisogamy see (Lehtonen and Parker 2014).
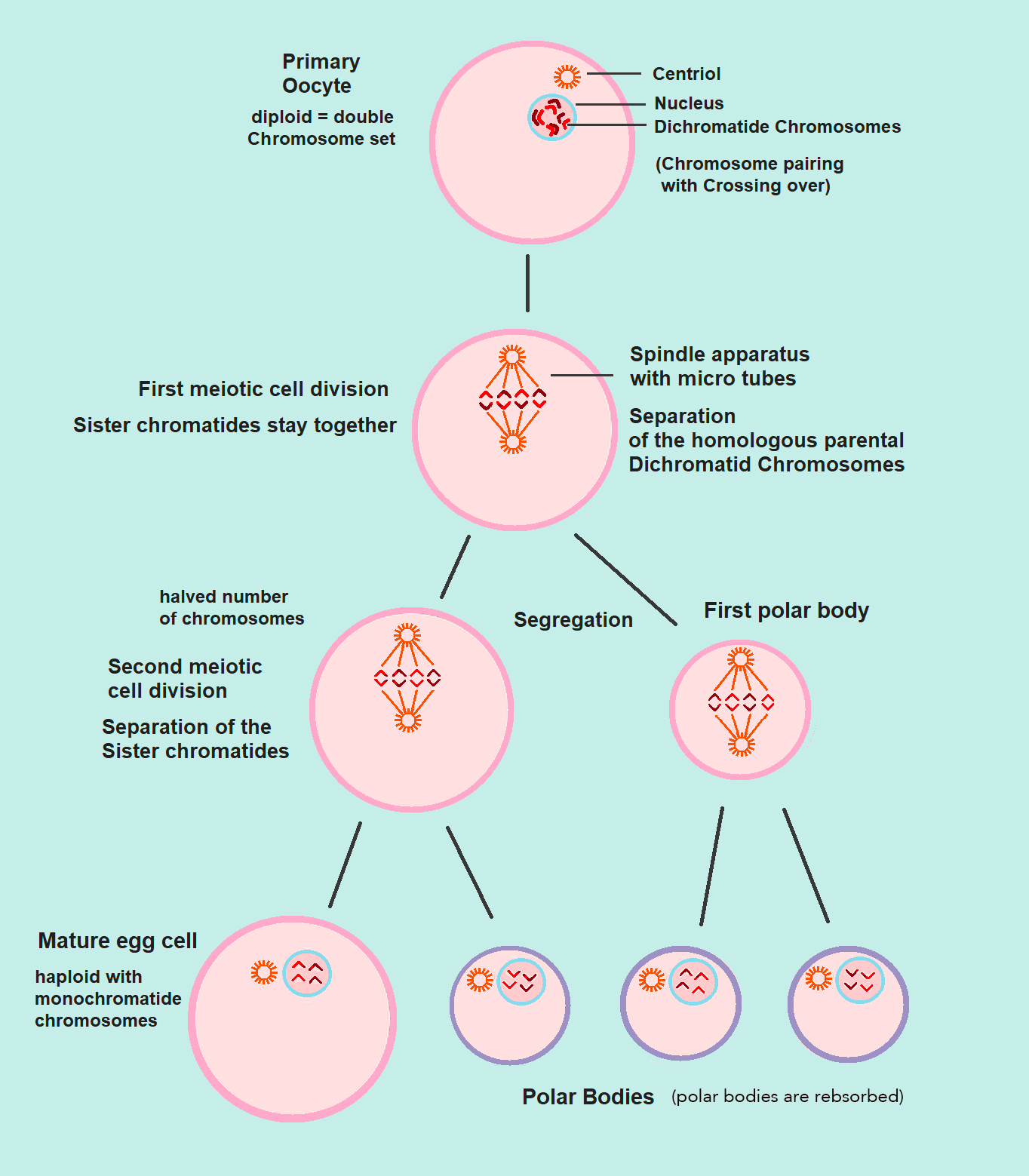
We can contrast gametogenesis in females and males (oogenesis with spermatogenesis, respectively) and see how oogenesis produces a single, large, immotile egg thanks to the unequal division of the cytoplasm in both MI and MII. This creates polar bodies that are reabsorbed, leaving just a single, extra-large egg. But in males, spermatogenesis has an equal division of the cytoplasm in both MI and MII producing four equally sized small, motile sperm (image below from Wikipedia. 2023. “Oogenesis” Wikimedia Foundation). The extra cytoplasm in the egg contributes to zygote development following fertilization.
Biological Sex Defined by Anisogamy
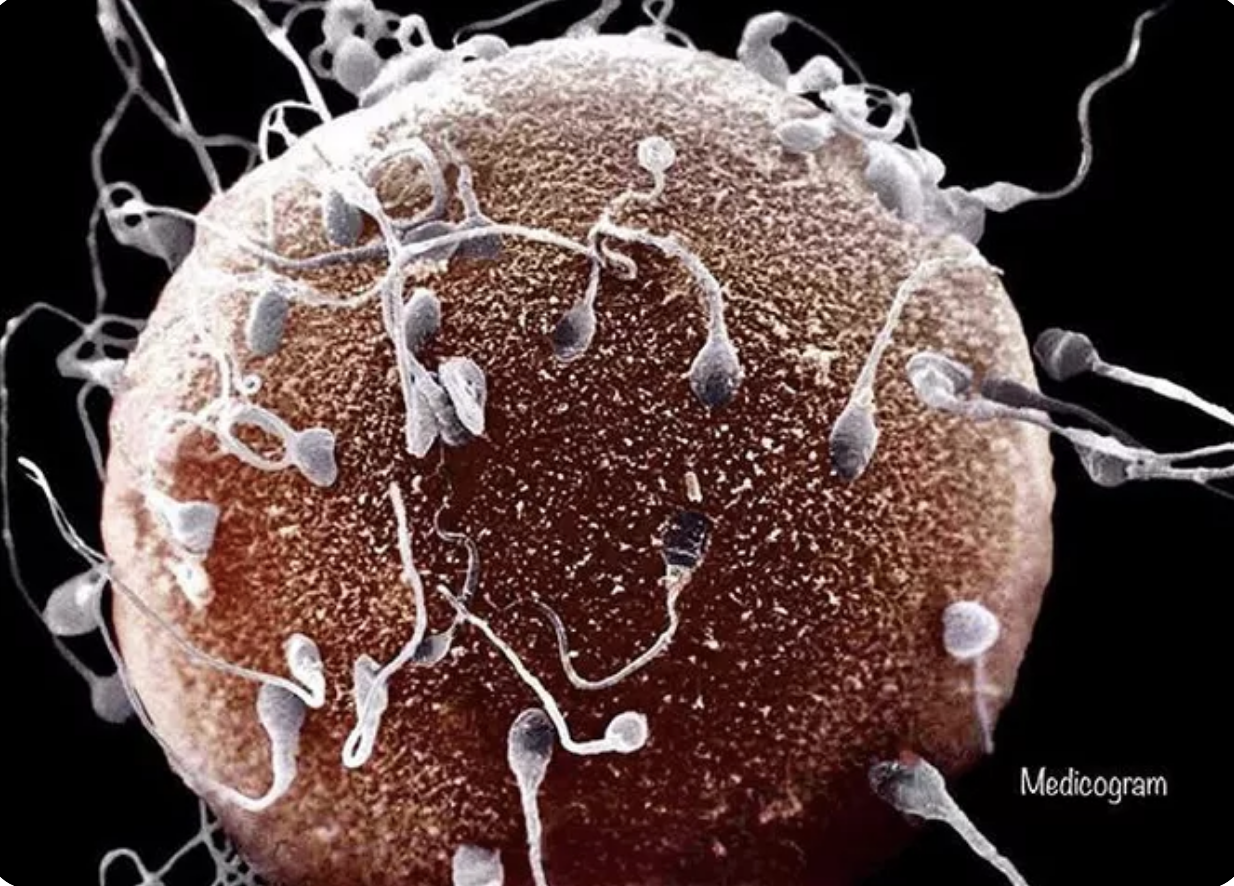
Although we often think of biological sex as the way to define multicellular organisms as either female or male based on their reproductive anatomy and physiology, it is more useful to look at the gamete size as the true way to define biological sex. Females produce macrogametes that remain stationary and males produce microgametes that move around (Parker, Baker, and Smith 1972); (Bell 1982); (Margulis and Sagan 1986). For example, in humans, the female egg diameter is greater than 0.1 mm, whereas the sperm, with its distinct flagellum allowing motility, has a much smaller size of just 5-7 μm long (pictured to the right). The asymmetry of anisogamous gametes leads to what (Parker 2014) refers to as the “sexual cascade” because it is the key to fundamentally establishing the basis for sexual dimorphism in body shape/size and differences in sexual behavior.
Why did sex evolve?
Asexual reproduction wins over sexual reproduction when all things are equal.
Asexual reproduction has many advantages over sexual reproduction and can be a highly fit way to reproduce, for example, some species of bdelloid rotifers (see below) have been successfully reproducing asexually for over 40 million years (Nowell et al. 2018). Asexual reproduction is significantly more efficient. It does not require finding a mate or the complex process of meiosis, which takes time and energy. Nor does it pose the obvious risks that mate attraction poses such as potential harm from convincing the mate to be receptive or risking obtaining disease from a mate. It also does not ruin the potentially fit genetic combination of alleles in a way that sexual reproduction does.
John Maynard Smith created a simple model testing the cost of sex (1978) that shows asexual clones multiply twice the rate of sexual entities. In the case of asexual reproduction, all individuals in the population are female and each female produces four offspring, which in turn produce four offspring. With sexual reproduction, two individuals (female and male) produce two offspring (male and female) and thus this asexual population increases at twice the rate of the sexual lineage. Thus, in a competition between asexual and sexual organisms, the asexual ones always outnumber the sexual ones. Several theoretical models and empirical experiments have supported the notion that asexual clones outcompete their sexual counterparts under constant conditions (Hamilton 1980); (Lively 1987); (Jokela et al. 1997).
The paradox of sex
Asexual reproduction has key advantages over sexual reproduction, but, of the one million multicellular animal species identified so far nearly all engage in sexual reproduction rather than asexual reproduction (Speijer et al. 2015). Why is sex so successful despite all its disadvantages? This is a paradox. It is clear that sexual reproduction produces a high level of genetic variation in offspring therefore natural selection favors this mode of reproduction over asexual reproduction. Since sex creates new genetic combinations selection of the more beneficial combinations under changing environmental conditions can increase fitness. Williams (1975) likened life to a lottery with sexually reproducing organisms able to buy multiple tickets compared to asexual organisms that only purchase a single ticket. Under this model, the sexual organisms increase the probability of their future offspring winning (i.e. surviving). But is that enough to cause the widespread obligate sexual reproduction seen in nature?
The key benefits of sexual reproduction
- sexual reproduction generates genetic diversity, increasing survival rates under changing conditions, and increasing reproductive fitness, which is defined as the ability of individuals to pass on their genes to subsequent generations;
- sexual reproduction helps rid populations of harmful genetic combinations.
Hypotheses on why sexual reproduction evolved
Red Queen Hypothesis
The Red Queen hypothesis (RQH), put forth by (Van Valen 1973) was inspired by Lewis Caroll’s Through the Looking Glass domineering character the Red Queen, who points out to a frustrated Alice, who has been running and running and getting nowhere that “it takes all the running you can do, to keep in the same place”. Van Valen was attempting to explain the observed constant rate of extinction supported by the fossil record with this analogy. The forward motion can seemingly get participants nowhere and Van Valen proposed that “every species does the best it can in the face of selection pressures, each species is part of a zero-sum game against other species” and “no species can ever win”. This is similar to what happens in coevolution which occurs when two directly interacting species affect one another’s evolutionary trajectory (Erlich and Raven 1975). The RQH was first used to explain the evolution of sex by Bell (1982) who noted that with two directly interacting species, sexual reproduction will enable them to coevolve rapidly to keep up with one another. Bell considered how genetic diversity oscillates (in models) in both parties involved in the coevolutionary “arms race” like Van Valen’s zero-sum game as illustrated in the diagram below.
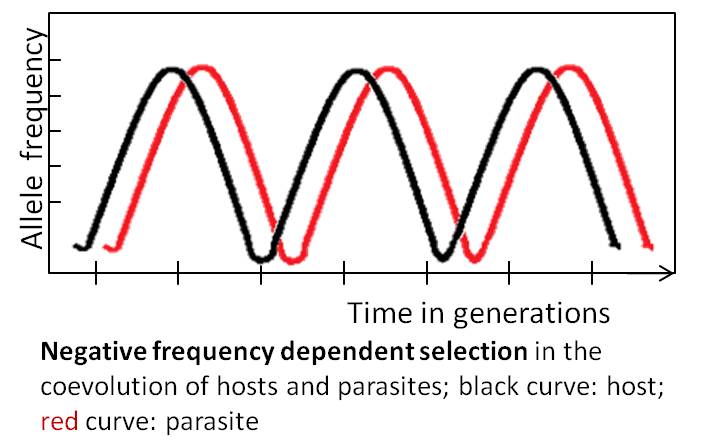
In the case of sexual reproduction, evidence suggests that host-parasite coevolution is a significant driver of sexual reproduction because it rapidly increases genetic combinations which can benefit a host by blocking infection from a parasite and conversely increase a parasite’s ability to infect its host (Lively 1996). Anything that decreases the likelihood of a host being infected by the parasite will have a selective advantage, and in turn, the parasite that increases its likelihood of infecting the host will have a selective advantage. This is one example of how interspecific interactions may drive sexual reproduction at the population level. For a more complete review of the RQH see Lively (Lively 2010).
Lively and his colleagues found support for the RQH in multiple experiments involving the New Zealand mud snail infected by a trematode parasite (Dybdahl and Lively 1998) [see below]. Additionally, Daphnia (water flea) and their parasite Pasteuria were studied in lake sediments, showing an increase in genetic diversity and virulence in the parasite over time (Decaestecker et al. 2007). Morran et al. (2011) also showed how sexually reproducing roundworms (Caenorhabditis elegans) were able to persist in the presence of coevolving bacterial (Serratia marcescens) parasites, whereas the asexual C. elegans were not, suggesting that sexual reproduction significantly increased their fitness in the presence of the parasite.
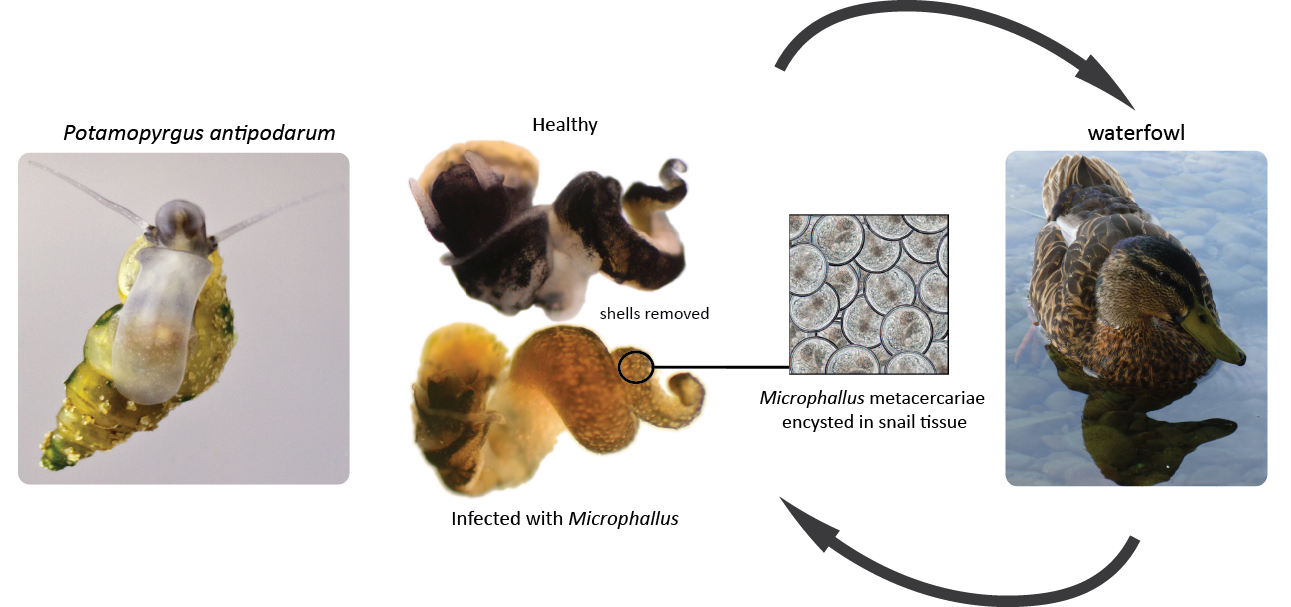
The New Zealand mud snail, Potamopyrgus antipodarum, is interesting because members of this species have both asexually and sexually reproducing females in the same populations found in different habitats. This snail is often infected by the trematode parasite in the genus Microphallus which uses a snail as an intermediate for its duck host (see image below). The trematode has a very strong impact on snail fitness, causing sterility in those heavily infected. Lively (1987) found this a great model to ask why sexual reproduction is predominant. Snails inhabiting different habitat types (stream and lake) were genotyped using allozymes, and measurements of parasite infection were used to directly compare sexual forms with asexual ones to see if there were any significant differences. Snails with rare genotypes had an advantage over parasite infection compared to those with more common genotypes. Laboratory experiments with parasite-exposed snails compared to non-exposed dissected control snails showed that once particular genotypes become more common in the population (increase in frequency) they become less resistant to infection. The prediction that sexual females would be more prevalent in sites with greater local parasite load was also supported by more recent research by Amanda Gibson involving the artificial inoculation of wild-caught snails and duck feces from Lake Alexandrina in NZ (Gibson, Xu, and Lively 2016), (image below depicting snail life-cycle from Gibson).
Q&A with Dr. Amanda Gibson
Assistant Professor at the University of Virginia

What made you want to study the RQH? I first heard about my PhD advisor Curt Lively’s work on the Red Queen Hypothesis when I was a freshman in college. My intro bio professor, Dr. Jill Miller, presented some of his data on the snail Potamopyrgus antipodarum and its trematode parasites. It wasn’t his results that stuck with me, but something Prof. Miller said about the approach of Curt and his lab – that they designed study after study to falsify the Red Queen Hypothesis. They hadn’t succeeded yet. So I was intrigued by the way they thought about problems. Once I was in the lab, I think ultimately it was the history of the study of the evolution of sex, and my graduate lab’s particular role in that history, that made me want to study the Red Queen Hypothesis. Evolutionary biologists have grappled with the problem of sex for decades now. Researchers generated a flurry of hypotheses to explain why sex is maintained, and Curt is one of the original scientists to compete those hypotheses against one another. I have the impression that no one expected the Red Queen to garner as much empirical support as it has.
It probably helped that in the first year of my Ph.D., Levi Morran, a postdoc in the lab, published a beautiful experiment showing that coevolving parasites can maintain outcrossing in laboratory populations of the nematode C. elegans. That study drove home to me how special parasites are as an evolutionary force – unlike abiotic features of the environment, parasites can reciprocally adapt, so host populations are never evolving toward a fixed optimum; the rules are always changing.
What did you like most about fieldwork? Fieldwork has brought me to beautiful, remote destinations that I might otherwise never have had the opportunity to visit. My doctoral research took me to New Zealand for six weeks every winter. I lived in a field station in Kaikōura, on the east coast of the South Island, where I found wonderful friends in the staff and researchers there.
Our team visited lakes all over the island to sample snails. Most of my work focused on Lake Alexandrina, where my advisor had studied these snails since 1985. In spending so much time at that lake year after year, working intensively, I got to know my surroundings in a special way. I knew which sites would have lots of snails, which were hard to get to, which had nice banks that were easy to sample, when the weather would make sampling a breeze, and where I’d find tons of parasites. I loved that sense of intimacy with these natural populations. Watching these organisms and environments for so long, I could tell what changed, and what stayed the same. That grounding in the field pointed me in the right direction as I tried to make sense of my data and helped me find the next question.
What did you find to be the most difficult thing about getting a PhD? Balance. I took 6 years to get my Ph.D., but I felt like it blew by. Each semester and each field season was packed with things I wanted to do. I loved that sense of excitement and urgency – it kept me motivated – but if I did it again, I would have set aside more time for the many other aspects of my life that I value.
Moving on is hard too! You become a real expert in your specific area during your Ph.D. I gained a lot of confidence as a scientist. Then, suddenly, you move on to your next thing, and it can feel like you’re starting over. I felt rejuvenated by learning new things in my postdoc, but I also found it difficult to step out of what had become my comfort zone and lose some of the momentum I’d had.
What do you enjoy most about your job now? Working closely with my students and trainees and watching them grow into independent, confident scientists. They raise questions and hypotheses that I know I would not have arrived at on my own, they have become experts in concepts or techniques that are totally new to me, and they regularly figure out how to do things I didn’t think were possible. I love that, as their advisor, I always get to be on their team.
Muller’s Ratchet Hypothesis
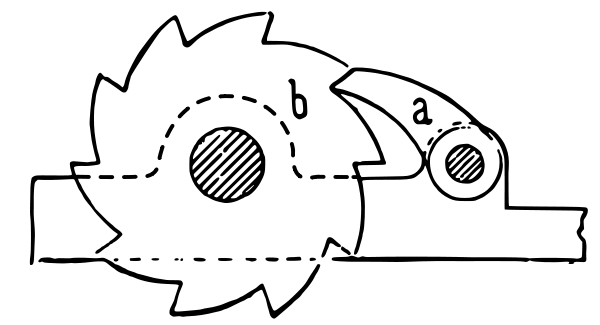
Mutations occur spontaneously and harmful ones can accumulate in the genome over time. Herman Muller (1964) recognized the implications of the accumulation of harmful mutations in asexual lineages over time and developed the analogy of a mechanical ratchet (a mechanical device that rotates in a single direction, preventing movement in the opposite direction, shown in the figure below). As each harmful mutation emerges the ratchet increases the mutational load and pushes an asexual organism closer to the inability to reproduce or even die. Experiments involving bacteriophage, ciliates, and E. coli, have all shown an increase in harmful mutational loads (Lin-Chao, Chen, and Wong 1992); (Lynch and Gabriel 1990); (Lenski 2017). Muller proposed the hypothesis that sexual reproduction evolved because it can reverse the “ratchet’s effect” by creating new genetic combinations, thereby purging harmful mutations. Thus sexual reproduction may be advantageous, particularly in small populations. Multiple models and computer simulations support this, particularly when they involve synergistic interactions (Hadany and Comeron 2008).
Limitations of Muller’s hypothesis
Sexual reproduction did not evolve from a single event, more likely it evolved independently numerous times. Margulis and Sagan (1986) contend a “series of historical accidents” resulted in meiotic cell division and that “selection has favored sexual organisms, but not because they were sexual”. Multiple mathematical models using unrealistic assumptions such as infinitely sized populations isolated from others under constant selection and experimental tests have involved over-simplified parameters (Otto and Lenormand 2002). Improved models would simulate realistic populations, incorporate more complex conditions, and account for randomness (Liow, Van Valen, and Stenseth 2011). For example, when recently testing the RQH MacPherson, Keeling, and Otto (2021) actually observed a decrease in genetic variation when they modeled host-parasite coevolution in a finite population rather than the expected increase produced by frequency-dependent selection.
Bdelliod rotifers (image on the left from Laine et al. 2020) comprise over 450 distinct species that inhabit freshwater ponds and lakes worldwide. They can survive under extreme conditions of starvation, extreme temperature, and prolonged desiccation by going dormant. Entire populations are all female, so it was thought they reproduced solely through apomixis. The diversity observed in these rotifers was hypothesized to come from horizontal gene transfer (the direct uptake of genes from other species). However, Veronika Laine and colleagues found strong support in their genome evolution for the hypothesis that these rotifers reproduce sexually on rare occasions. Laine, Sackton, and Meselson (2022) show that these rotifers are sexual only when it is essential, but the RQH cannot explain why they continue to reproduce sexually because “Bdelloids gain freedom from having to co-evolve with biological antagonists by their ability to survive prolonged starvation, extremes of temperature and exposure to toxic conditions lethal to other taxa”. The evolution of sex is likely much more complicated than either of these hypotheses can explain.
Q&A with Dr. Veronika Laine, Ph.D.
Postdoctoral Researcher at the Finland Museum of Natural History
What drew you to using genomics to answer evolutionary biology questions? I have always been interested in how different species adapt to changing environments and which genes play a part in adaptation. I especially want to link the molecular changes to individual phenotypes to population and ecosystem levels. Since I am also a computer geek, the technology and tools used in genomics make the field really interesting and always evolving. There are always new tricks to be learned.
What did you find to be the most difficult thing about getting a PhD? I think the most challenging part was project and time management. Especially during the end part of the Ph.D. when deadlines were approaching and tasks piling up, learning these management skills was crucial. Luckily there was a course about these topics in the last year of my Ph.D. and this saved my research project. These skills are important for the future as well.
What do you think is the biggest challenge women in evolutionary biology face? Things have gotten better for women in science, but many things persist like the glass ceiling in academia preventing women from climbing up the career ladder. Fortunately, there have been measures taken to make academia more diverse. Diversity in researchers helps us to make better science.
What did you like most about your job? Every day I learn something new, and I love how I can use my creativity in solving biological questions. I have also met so many awesome people, who have helped and inspired me. The colleagues around me have made this the best job ever.
In summary, the evolution of sex is a fascinating and complex area of study in biology. It involves the interplay of various selective pressures, trade-offs, and genetic mechanisms. While there are many theories and hypotheses, ongoing research continues to shed light on the intricacies of sexual reproduction and its evolution. In the next chapter, we will dive deeper into the genetic mechanisms involved in establishing biological sex.
Think, Pair, Share
How do you think Miriam Minken’s life would have been different if she were treated equally? How might it have changed the course of IVF research?
Cell division is essential for reproduction but not for sex, explain.
Why can’t biologists agree on the driver(s) of sexual reproduction?
Given the costs, is sexual reproduction worth it?
Deeper Questions
Parker defines biological sex as it relates to gamete size. Is this an adequate definition? why or why not?
If you could design the perfect experiment to test how essential sex is for evolution what would it entail?
Sexual reproduction is responsible for much of the diversity of life on the planet but not all. In what ways does diversity arise without sex?
What reasons might there be for extremophile rotifers to utilize facultative sexual reproduction rather than obligate sexual reproduction?
Key Terms
Anisogamy
Apomixis
Automixis
Diploid
Diplotonic
Diplohaplotonic
Gamete
Germ cells
Haploid
Haplotonic
Homologous chromosomes
Meiosis
Mitosis
Muller’s Ratchet Hypothesis
Natural selection
Oogenesis
Recombination
Red Queen Hypothesis
Sex
Sexual cascade
Somatic cells
Syngamy
Zygote
References
Avery, O. T., C. M. Macleod, and M. McCarty. 1944. “Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumoccocus type III.” The Journal of Experimental Medicine 79 (2): 137–58. https://doi.org/10.1084/jem.79.2.137.
Bateson W. 1901. “Problems of heredity as a subject for horticultural investigation.” J R Horticultural Society 25:54–61.
Bell, G.. 1982. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Berkeley: University of California Press.
Boveri, T. 1904. “Noch Ein Wort Über Seeigelbastarde.” Archiv Für Entwicklungsmechanik Der Organismen 17: 521–25.
Carothers, E. E.. 1917. “Mologous chromosomes as found in two genera of acrididae (Orthoptera).” Journal of Morphology 28: 445.
Crews, D., and K. T. Fitzgerald. 1980. “‘Sexual’ Behavior in Parthenogenetic Lizards (Cnemidophorus).” PNAS 77 (1): 499–502. https://doi.org/10.1073/pnas.77.1.499.
Decaestecker, E., S. G., J. A. M. Raeymaekers, R. Stoks, L. Van Kerckhoven, D. Ebert, and L. Meester. 2007. “Host–Parasite ‘Red Queen’ Dynamics Archived in Pond Sediment.” Nature 450 (7171): 870–73. https://doi.org/10.1038/nature06291.
Deneke, Victoria E., Andreas Blaha, Yonggang Lu, Johannes P. Suwita, Jonne M. Draper, Clara S. Phan, Karin Panser, et al. 2024. A Conserved Fertilization Complex Bridges Sperm and Egg in Vertebrates.” Cell 187 (25): 7066-7078.e22. https://doi.org/10.1016/j.cell.2024.09.035.
Derbès, A. A. 1847. “Observations Sur Le Méchanisme et Les Phenomènes Qui Accompagnent La Formation de l’Embryon Chez l’Oursin Comestible,” 80–98.
Dusenbery, D. B., and T. W. Snell. 1995. “A Critical Body Size for Use of Pheromones in Mate Location.” Journal of Chemical Ecology 21 (4): 427–38. https://doi.org/10.1007/BF02036740.
Dusenbery, D. B. 2002. “Ecological Models Explaining the Success of Distinctive Sperm and Eggs (Oogamy).” Journal of Theoretical Biology 219 (1): 1–7.
Dybdahl, M. F., and C. M. Lively. 1998. “Host-parasite coevolution: evidence for rare advantage and time-lagged selection in a natural population.” Evolution; International Journal of Organic Evolution 52 (4): 1057–66. https://doi.org/10.1111/j.1558-5646.1998.tb01833.x.
Erlich, P. R., and P. H. Raven. 1975. “Butterflies and Plants: A Study in Coevolution” Evolution 18: 586–608.
Ferris, P. J., and U. W. Goodenough. 1994. “The Mating-Type Locus of Chlamydomonas Reinhardtii Contains Highly Rearranged DNA Sequences” Cell 76 (6). https://doi.org/doi: 10.1016/0092-8674(94)90389-1.
Fol, H.. 1877. “Sur Le Commencement de l’h´enog´enie Chez Divers Animaux.” 6: 145–69.
Futuyma, D.. 2009. “Natural Selection and Adaptation.” In “Evolution”, (Sunderland MA, Sinauer Associates) 279–301.
Gibson, A., J. Xu, and C. M. Lively. 2016. “Within-Population Covariation between Sexual Reproduction and Susceptibility to Local Parasites” Evolution 70 (9): 2049–60.
Grant, R. B., and P.R. Grant. 2003. “What Darwin’s Finches Can Teach Us about the Evolutionary Origin and Regulation of Biodiversity.” BioScience 53 (10): 965–75. https://doi.org/10.1641/0006-3568(2003)053[0965:WDFCTU]2.0.CO;2.
Hadany, L., and J. M. Comeron. 2008. “Why Are Sex and Recombination So Common?” Annals of the New York Academy of Sciences 1133 (1): 26–43. https://doi.org/10.1196/annals.1438.011.
Hamilton, W.. 1980. “Sex versus Non-Sex versus Parasite” Oikos 35 (2): 282–90.
Hanschen, E., M.D. Herron, J. Wiens, H. Nozaki, and R. Michod. 2018. “Multicellularity Drives the Evolution of Sexual Traits” The American Naturalist 192 (3): 287–399.
Heesch, S., M. Serrano-Serrano, J. Barrera-Redondo, R. Luthringer, A.F. Peters, C. Destombe, J. M. Cock, et al. 2021. “Evolution of Life Cycles and Reproductive Traits: Insights from the Brown Algae” Journal of Evolutionary Biology 34: 992–1009.
Hertwig, O.. 1876. “Beitrage zur Kenntniss der Bildung, Befruchtung und Theilung des thierischen Eies.” 1: 347–432.
Hojsgaard, D., and E.Hörandl. 2019. “The Rise of Apomixis in Natural Plant Populations.” Frontiers in Plant Science 10 (2019): 358.
Jokela, J. , C. M. Lively, M. F. Dybdahl, and J. A. Fox. 1997. “Evidence for the cost of sex in a freshwater snail potamopyrgus antipodarium” Ecology 78 (2): 452-460.
Laine, V. N., T. B. Sackton, and M. Meselson. 2022. “Genomic Signature of Sexual Reproduction in the Bdelloid Rotifer Macrotrachella Quadricornifera.” Genetics 220 (2). https://doi.org/10.1093/genetics/iyab221.
Lehtonen, J., H. Kokko, and G. A. Parker. 2016. “What Do Isogamous Organisms Teach Us about Sex and the Two Sexes?” Philosophical Transactions of the Royal Society B: Biological Sciences 371 (1706): 20150532. https://doi.org/10.1098/rstb.2015.0532.
Lehtonen, J., and G. A. Parker. 2014. “Gamete Competition, Gamete Limitation, and the Evolution of the Two Sexes” Molecular human reproduction 20 (12): 1161–68.
Lenski, R.. 2017. “Experimental Evolution and the Dynamics of Adaptation and Genome Evolution in Microbial Populations” The ISME journal 11: 2181–94. https://doi.org/10.1038/ismej.2017.69.
Lin-Chao, S., Wen-Tsuan Chen, and Ten-Tsao Wong. 1992. “High Copy Number of the pUC Plasmid Results from a Rom/Rop-Suppressible Point Mutation in RNA II.” Molecular Microbiology 6 (22): 3385–93. https://doi.org/10.1111/j.1365-2958.1992.tb02206.x.
Liow, L. H., L. Van Valen, and N. C. Stenseth. 2011. “Red Queen: From Populations to Taxa and Communities.” Trends in Ecology 26 (7): 349–58. https://doi.org/10.1016/j.tree.2011.03.016.
Lively, C.M. 1987. “Evidence from a New Zealand Snail for the Maintenance of Sex by Parasitism.” Nature 328 (6130): 519–21. https://doi.org/10.1038/328519a0.
———. 1996. “Host-Parasite Coevolution and Sex” Bioscience 46 (2): 107–14.
———. 2010. “A Review of Red Queen Models for the Persistence of Obligate Sexual Reproduction” Heredity 101 (suppl_1): S13–20.
Lynch, M., and W.Gabriel. 1990. “Mutation Load and the Survival of Small Populations” Evolution 44 (7): 1725–37.
MacPherson, A., M.J. Keeling, and S. P. Otto. 2021. “Feedback between Coevolution and Epidemiology Can Help or Hinder the Maintenance of Genetic Variation in Host-Parasite Models.” Evolution 75 (3): 582–99. https://doi.org/10.1111/evo.14165.
Margulis, L., and D. Sagan. 1986. Microcosmos: Four Billion Years of Evolution from Our Microbial Ancestors. Summit Books.
Marsh, M., and W. Ronner. 2019. The Pursuit of Parenthood: Reproductive Technology from Test-Tube Babies to Uterus Transplants. Johns Hopkins University Press.
Maynard Smith, J. 1978. The Evolution of Sex. Cambridge University Press.
Mendel, G. 1866. “Versuche Über Pflanzen-Hybriden.” Im Verlage Des Vereines. https://doi.org/10.5962/bhl.title.61004.
Morgan, T. H. 1911. “Random Segregation Versus Coupling in Mendelian Inheritance,” Science 34 (873): 384-384.
Morran, L.T., O.G. Schmidt, I. A. Gelarden, R. C. Parrish, and Curtis M. Lively. 2011. “Running with the Red Queen: Host-Parasite Coevolution Selects for Biparental Sex” Science 333 (6039): 216–18.
Muller, H.. 1964. “The Relation of Recombination to Mutational Advance” Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 1: 2–9.
Nowell, R. W., P. Almeida, C. G. Wilson, T. P. Smith, D. Fontaneto, A. Crisp, G. Micklem, A. Tunnacliffe, Chiara Boschetti, and T. Barraclough. 2018. “Comparative Genomics of Bdelloid Rotifers: Insights from Desiccating and Nondesiccating Species” PLoS biology 16 (4). https://doi.org/10.1371/journal.pbio.2004830.
Otto, S. P., and T. Lenormand. 2002. “Resolving the Paradox of Sex and Recombination.” Nature Reviews Genetics 3 (4): 252–61. https://doi.org/10.1038/nrg761.
Parker, G. A. 2014. “The Sexual Cascade and the Rise of Pre-Ejaculatory (Darwinian) Sexual Selection, Sex Roles, and Sexual Conflict.”Cold Spring Harbor Perspectives in Biology 6(10): p.a017509.
Parker, G. A., R. R. Baker, and V. G. F. Smith. 1972. “The Origin and Evolution of Gamete Dimorphism and the Male-Female Phenomenon.” Journal of Theoretical Biology 36: 529–33.
Rock, J., and M. Menkin. 1944. “In Vitro Fertilization and Cleavage of Human Ovarian Eggs” Science 100 (2588): 105–7. https://doi.org/DOI: 10.1126/science.100.2588.10.
Rodriguez, S. 2015. “Watching the Watch-Glass: Miriam Menkin and One Woman’s Work in Reproductive Science, 1938–1952” Women’s Studies 44 (4): 451–67.
Sato, A., C. O’hUigin, F. Figueroa, P. R. Grant, B. R. Grant, H. Tichy, and J. Klein. 1999. “Phylogeny of Darwin’s Finches as Revealed by MtDNA Sequences.” PNAS 96 (9): 5101–6. https://doi.org/10.1073/pnas.96.9.5101.
Speijer, D., Lukeš, J. and Eliáš, M., 2015. “Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life.” PNAS 112 (29):8827-8834.
Sturtevant, A.. 1913. “The Linear Arrangement of Six Sex-Linked Factors in Drosophila, as Shown by Their Mode of Association” Journal of Experimental Zoology 14: 43–59.
Sutton, W.. 1902. “On the Morphology of the Chromosome Group in Brachystola Magna” The Biological Bulletin 4 (1): 24–39.
———. 1903. “The Chromosomes in Heredity,” The Biological Bulletin 4 (5): 231-250.231–50.
Van Valen, L.. 1973. “A New Evolutionary Law Evolutionary Theory.”
Weismann, A.. 1885. “The Continuity of the Germ-Plasm as the Foundation of a Theory of Heredity.” Essays upon Heredity and Kindred Biological Problems, 163–254.
Wilkins, A. S., and R. Holliday. 2009. “The Evolution of Meiosis From Mitosis.” Genetics 181 (1): 3–12. https://doi.org/10.1534/genetics.108.099762.
Williams, G.C. 1975. Sex and Evolution. Princeton University Press.
Suggested Readings
“The Red Queen: Sex and the Evolution of Human Nature” by Matt Ridley – This book explores the role of sexual reproduction in the evolution of complex organisms, including humans. Matt Ridley discusses various aspects of sexual selection and its impact on human behavior and biology.
“Why Sex Matters: A Darwinian Look at Human Behavior” by Bobbi S. Low – Bobbi Low takes a scientific approach to examine the evolutionary basis of human sexual behavior, covering topics such as mate choice, sexual conflict, and reproductive strategies.
“The Evolution of Beauty: How Darwin’s Forgotten Theory of Mate Choice Shapes the Animal World—and Us” by Richard O. Prum – This book explores Charles Darwin’s theory of sexual selection and how it has influenced the development of extravagant traits and behaviors in animals, including humans.
“The Dawn of the Deed: The Prehistoric Origins of Sex” by John A. Long – Paleontologist John A. Long delves into the evolutionary history of sexual reproduction, tracing its origins in ancient aquatic organisms and its subsequent diversification.
“The Social Conquest of Earth” by Edward O. Wilson – While not exclusively about the evolution of sex, this book by renowned biologist Edward O. Wilson delves into the evolution of social behavior, which is closely tied to reproductive strategies and mate selection.
“Sperm Wars: Infidelity, Sexual Conflict, and Other Bedroom Battles” by Robin Baker – Robin Baker delves into the evolutionary biology of human sexuality, particularly the role of sperm competition and sexual conflict in shaping reproductive strategies.
Interesting Podcast
TED radio hour How birds, bees and bugs multiply and thrive
