8 Pregnancy
STUDENT LEARNING OUTCOMES
At the end of this chapter you should be able to:
- Describe the process of fertilization
- Compare the contribution of egg and sperm in the zygote
- Describe implantation
- Discuss the role of the placenta during pregnancy
- Describe how pregnancy test kits work
- Explain how the due date for pregnancy is determined
- List the changes in a woman’s body that occur during pregnancy
- Recall the complications that may arise during pregnancy
- Recognize the importance and limitations of prenatal care
- Describe the role midwives can play
- State how pain can be dealt with during labor
- Explain breastmilk and the importance of breastfeeding
- Discuss hypotheses about the evolution of mammary glands
Pregnancy is a significant and complex life event that has profound physical, emotional, and social implications
Thanks, mom! We can all attribute our existence to our mother and to the amazing phenomenon called pregnancy. It is a period of major physical and emotional change for women and thus pregnancy is a big deal! Pregnant women are in great need of social support and although it is a normal physiological process complications from pregnancy are the leading cause of death globally among adolescent girls aged 15-19 years. Furthermore, the United States has the highest maternal mortality rates among developed countries, particularly for women of color (OECD 2023). Being informed and having the ability to process the information, ask questions, and have the ability to discuss information with a healthcare professional before a decision regarding pregnancy is made is ideal (Moore 2016). In this chapter, the stages of pregnancy from fertilization to birth are discussed.
From conception to 12 weeks
Fertilization
Fertilization, first described in detail from studying sea urchins (Hertwig 1876), occurs when the egg and sperm fuse. However, compared to sea urchins, mammals experience internal fertilization making understanding the process challenging. Researchers are still working to unlock some of the mysteries surrounding the process. In humans, coitus (the penetration of the vagina by the penis) may result in the ejaculation of sperm, of which approximately 200 from the initial 280 x 106 sperm ejaculated into the vagina reaches the ampullary region of the oviduct (Boitrelle et al. 2021). Intrauterine insemination (IUI) may also be used whereby a minimum of 6.7 million sperm are inserted into the uterus for successful fertilization, using a small catheter (Higdon et al. 2008).
Recall that the follicle ruptures during ovulation and the mature ovum (egg) is released from the ovary into the peritoneal cavity where the fimbriae, finger-like projections at the end of the fallopian tube (oviduct) pick up the released egg. There it awaits the arrival of sperm which swim toward the oviducts. Sperm are assisted by the muscular activity of the uterus and the ciliary beating of the oviduct. As sperm travel toward the oviduct they must experience capacitation (incubation) to undergo the acrosomal reaction. The acrosomal reaction is an ectocytotic event (contents of a cell vacuole are released to the exterior through fusion of the vacuole membrane with the cell membrane) that makes it possible for the sperm to fuse with the egg. The acrosome is located in the anterior portion of the sperm head and contains enzymes that assist in the penetration of the extracellular matrix (zona pellucida) of the egg (see figure below).
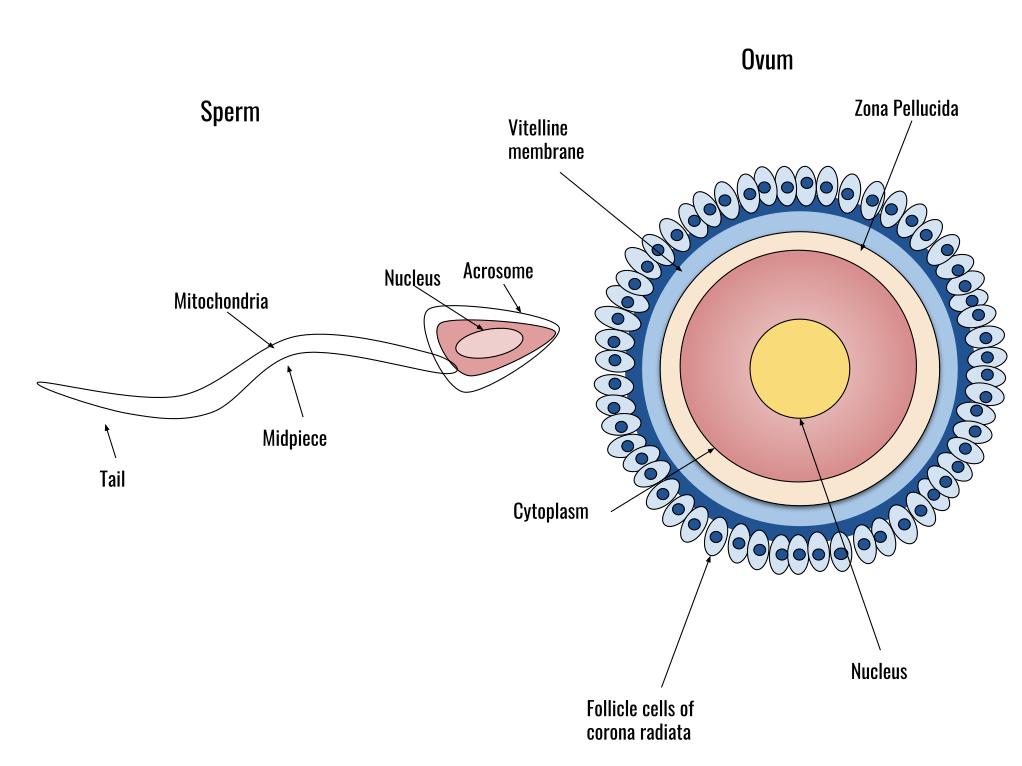
Data suggest that both the ovary and egg secrete chemotactic substances to help attract the sperm to the egg (Sun et al. 2005). Once the sperm reaches the egg the zona pellucida binds the sperm with the assistance of ZP3, a glycoprotein on the zona pellucida that binds to sperm along with additional proteins. The binding of ZP3 along with progesterone induces the acrosomal reaction. Once the acrosomal reaction happens fusion of the sperm cell membrane with the egg cell membrane occurs.
Polyspermy, the fusing of more than one sperm to an egg, can result in the lethal problem of triploidy (having an extra set of chromosomes). Polyspermy is blocked in mammals by three mechanisms according to Evans (2020):
Block 1) Sperm travel through the uterotubal junction where they are exposed to oviducal epithelial cells/secretions that inhibit many sperm;
Block 2) Exocytosis of cortical granules from the cortex of the egg inhibits zona pellucida from supporting further sperm binding; and
Block 3) Membrane block induced by increased Ca2+ in the cytosol of the egg (mechanism not yet understood).
Egg activation
Egg activation occurs after fertilization has taken place, following the fusion of the sperm and egg membranes. An increase in Ca2+ concentration (the calcium wave) in the cytoplasm triggers the resumption of meiosis in the mature oocyte and activates the development of the embryo. The male pronucleus enlarges while the oocyte nucleus completes its second meiotic division. Each pronucleus replicates its DNA and they migrate toward one another. They meet and the chromatin condenses into chromosomes that orient themselves on a common mitotic spindle. The sperm contributes a pair of centrioles that are duplicated, one of each moves to each pole of the egg cell, replacing the those of the egg cell. A series of metabolic events are also triggered thanks to the calcium wave.
Rearrangement of the egg cytoplasm
The haploid male pronucleus is necessary for egg activation and contribution to the diploid genome but the rest of the sperm plays no major role in embryogenesis. The egg contains key morphogenic determinants that become segregated into specific cells during cleavage. These lead to the activation and/or repression of specific genes and are known as maternal factors, also known as maternal effect genes. How maternal effect genes work was first described by Nüsslein-Volhard et al. (1980) in Drosophila however it took another 20 years before similar genes were described in mammals (Christians et al. 2000). Christine Nüsslein-Volhard earned the Nobel Prize in Physiology or Medicine in 1995 for her research on the genetics of embryonic development in Drosophila. See her Nobel Laureate website.
The embryo and cleavage
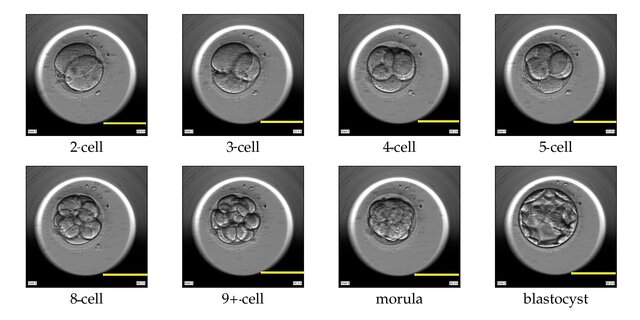
All animal species undergo cleavage, a series of mitotic divisions whereby the egg cytoplasm is divided into numerous smaller nucleated cells (image of cleavage below). In mammals, the first cleavage begins about a day after fertilization and happens as the cilia in the oviduct push the embryo toward the uterus. A key distinction between mammals and all other animals is that they go through a 16-cell morula stage consists of a small group of internal cells surrounded by a larger group of external cells. In non-mammalian animals, embryonic development varies significantly, and they may not go through a morula stage like mammals. For example, birds do not form a morula instead they form a blastoderm which is a disc-shaped structure that forms on top of the yolk. From the morula, most of the external cells become the trophoplast whose cells will form the embryonic portion of the placenta, the chorion. The inner cell mass gives rise to the embryo and its associated yolk sac, allantois, and amnion. At the 32-cell stage, the structure is called the blastocyst (see figure below) and by the 64-cell stage, the inner cell mass and trophoblast become separate cell layers. The cells in the inner cell mass are pluripotent and are called embryonic stem cells; they will ultimately give rise to each of the 200 cell types in our bodies. The trophoblast cells will be involved in the uterine invasion (described below) and the formation of the extraembryonic membranes that are a feature of all amniotes.
Gastrulation in mammals
Approximately 14 days post fertilization the embryo begins gastrulation whereby the blastocyst is transformed into a gastrula. Tam and Behringer describe gastrulation in the mouse (Tam and Behringer 1997): during gastrulation the body plan is formed to serve as a “blueprint for the subsequent morphogenesis of the embryo”, making the anterior-posterior axis obvious and creating the three germ layers: ectoderm, mesoderm, and endoderm through cell proliferation and differentiation. The germ layers are then “partitioned into domains of progenitor cells that give rise to the major tissue types”. As for humans, recent research by Tyser et al. (2021) found that “the signaling pathways of gastrulating cells transitioning from epiblast to mesoderm was broadly conserved between humans and the mouse…” with differences in regulation.
Implantation of the blastocyst
Humans are eutherian mammals, a group characterized by the development of the placenta that allows for advanced development as compared to non-eutherian mammalian monotremes (egg laying, e.g. the echidna) or marsupials (complete development in a pouch, e.g. opossum). In eutherians, implantation occurs when the embryo attaches itself to the endometrial surface of the uterus and invades the epithelium and the placenta will form. This is referred to as a “proinflammatory process” and is reviewed by Kim and Kim (2017). It is a time-sensitive event with a specific “window of implantation” where the blastocyst and receptive uterus communicate through a “synchronized molecular dialogue”. For humans, this window lasts from days 16-22 of a 28-day cycle when the endometrium has been primed to accept the blastocyst. This is a critical time during pregnancy and most pregnancy losses (miscarriages) occur soon after implantation.
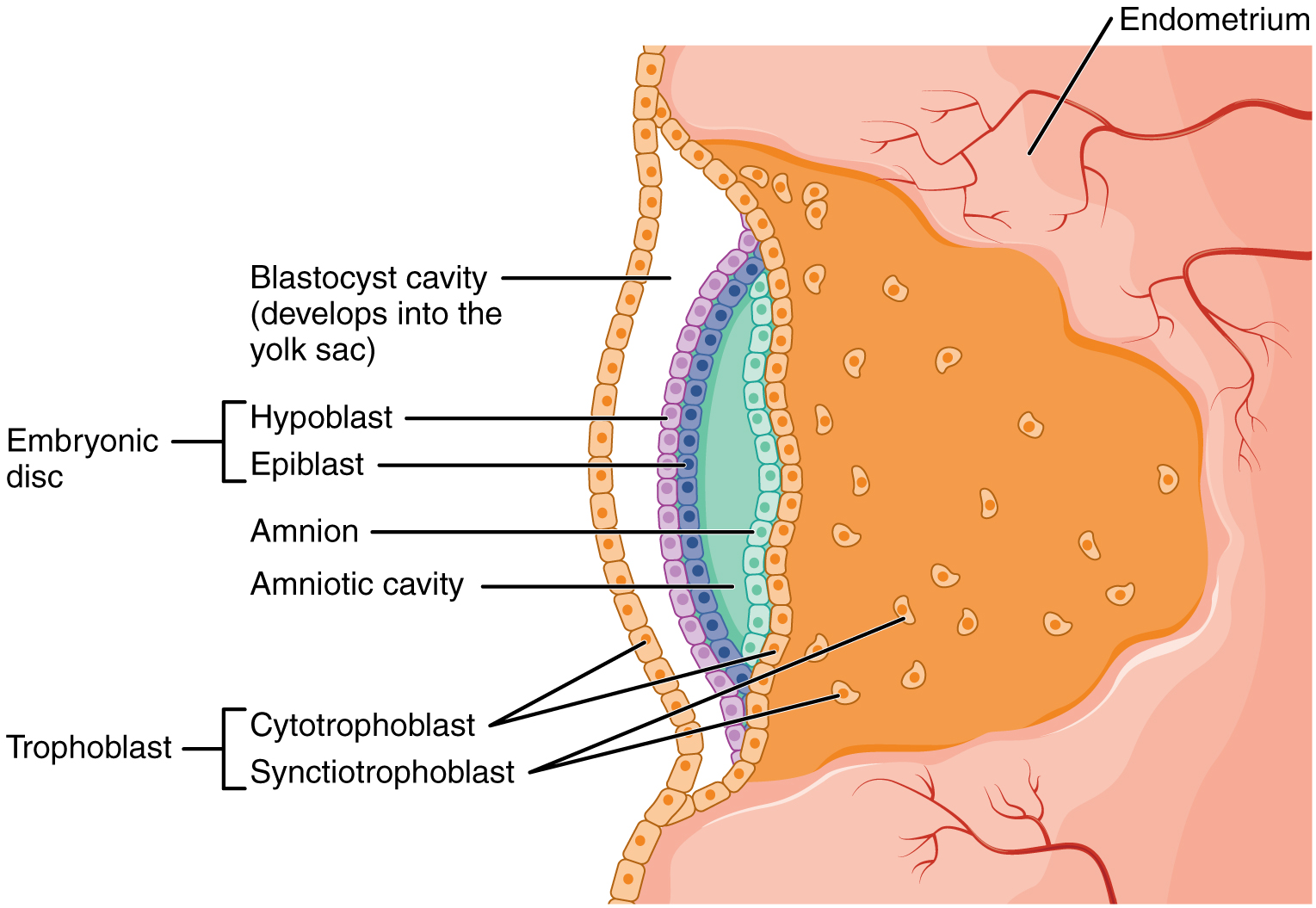
Approximately 1-3 days after the morula enters the uterine cavity implantation is initiated with the loss of the zona pellucida. The morula becomes a blastocyst that adopts a more irregular surface with microvilli. The blastocyst touches the implantation site adhering itself at the uterine epithelium creating a pro-inflammatory reaction mediated by prostaglandins. It is during this process that the blastocyst differentiates into an inner cell mass (embryoblast) and trophoblast (pictured above). Cytokines (regulatory peptides) and growth hormones interact between the trophoblast and uterus during attachment and both cell adhesion molecules and integrins (transmembrane glycoproteins) play a key role in successful adhesion of the trophoblast to the endometrial epithelium.
According to Kim and Kim (2017) once attached to the epithelium the trophoblast cells become cytotrophoblast cells and syncytiotrophoblast cells. The cytotrophoblast cells produce enzymes so the syncytiotrophoblast cells can invade and migrate into the thickened and vascularized endometrial lining, penetrating the basement membrane (pictured above). The syncytiotrophoblast cells destroy and reconstruct the maternal spiral arteries to increase the blood supply and oxygen for the fetus. The syncytiotrophoblast cells create the placental villi with the aid of growth factors, cytokines, and enzymes while modulating the maternal immune response. These villi will begin to establish fetal-maternal communication, orchestrating the complex biomolecular interactions that result in the formation of the placenta.
The placenta
The placenta is comprised of the chorion, amnion, and umbilical cord and serves as an interface between the mother and developing fetus, facilitating the exchange of oxygen, nutrients, and waste products and assisting in maternal physiological adaptations to pregnancy. The umbilical cord is a critical link between the developing fetus and the maternal circulation during pregnancy, and it ensures the exchange of substances necessary for fetal growth and well-being. It contains two arteries and one vein. The two umbilical arteries carry deoxygenated blood and waste products away from the fetus to the placenta, while the umbilical vein carries oxygenated blood and nutrients from the placenta to the fetus. The fluid-filled amnion (amniotic sac) surrounds the embryo providing a protective cushion. The placenta develops as the pregnancy progresses (see image below).
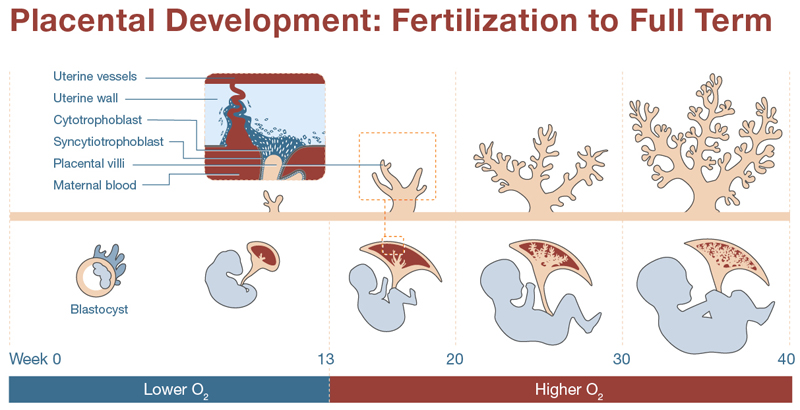
In a review of the evolution of the placenta, the organ is described as “the least conserved and the most rapidly evolving mammalian organ” (Roberts, Green, and Schulz 2016). Several genes involved in placental development are imprinted which means that they are expressed based on parent of origin through epigenetic control mechanisms (Hanna 2020). The outer layer of the trophoblast forms the chorion which produces human chorionic gonadotropin (hCG) that plays a crucial role in producing progesterone maintaining early pregnancy until the placenta can take over hormone production. Issues that may arise impact the development of the placenta can lead to severe problems for the embryo and often underlie miscarriages (Rossant and Cross 2001). A serious effort to better understand the placenta (the Human Placenta Project) was launched in 2015 by the NIH setting aside over $40M in funding (Reardon 2015).
As discussed above, the placenta is a critical link between the fetus and mother and when it does not develop properly there can be inadequate blood supply (ischemia). Dr. Laura Clamon Schulz and associates examined the effect of undernutrition during early gestation on placental development in mice and found food restriction from three weeks before mating through 11.5 days post-conception alters placental structure (Van Gronigen Case et al. 2021).
Q&A with Dr. Laura Clamon Schulz, Ph.D.

Associate Professor of Obstetrics, Gynecology and Women’s Health,
Director ObGyn Research Success Core, Director SOM OneHealth Biorepository at the University of Missouri
What drew you to studying women’s reproductive health and specifically the placenta? In college, I fell in love with a class in comparative physiology. I thought (and still think) that studying the diversity of animal physiology, and the variety of solutions animals have developed to survive in their particular environments, is a powerful tool for understanding how our own bodies function. And so I joined the laboratory of Dr. Janice Bahr in graduate school for the chance to work with pregnancy in American Black Bears, and that’s where I became a reproductive physiologist. After a postdoc studying pregnancy in bats, I moved to Missouri, in part for family reasons, and got the opportunity to do a second postdoc with a National Academy of Sciences member, Mike Roberts. As it turns out, I stuck with studying pregnancy and questions about how the placenta forms, even though I use laboratory mice and cell culture systems, and try to address health-related questions. I switched largely because of where I found job and funding opportunities, but I have become passionate about being able to contribute to pregnancy health.
What have you enjoyed most about your career? I love the thrill of figuring out something new – whether it is just getting a new technique to work, or realizing how all the data fit together to reveal a new truth. But to be honest, those joys are fleeting, and the most sustained joy comes from mentoring and seeing my student’s accomplishments and their growth is incredibly fulfilling.
How do you see the career path for women in science has changed since you began and what do you hope for the future? It is hard to compare, as I am not a student now, and I was not a mid-career scientist then. That is, I can’t tell if the reductions in casual sexism that I’ve experienced are because of my age or because things have gotten better, but I hope it’s the latter. At my University I see more women in leadership positions now. I recall there being only one woman professor in my undergraduate Biology department in the 90s, just my graduate advisor in her department, and just one female department chair in the School of Medicine when I arrived here in the late 2000s
What advice do you have for future women scientists? I would urge women not to be scared off because they want to have a family and a scientific career. I don’t want to minimize anyone else’s struggles, and it hasn’t been without its challenges, but I think the perception of work-life balance issues has been more of a barrier than actual balance issues in my career. Granted, it’s much easier if one has an equal partner. I also would urge future scientists to craft a professional demeanor that feels true to themselves. As a woman, you will always be told you are too something (quiet, loud, serious, silly) and it’s better to spend your time trying to be a good scientist and colleague than to please all of them, which is impossible anyway.
Organogenesis
By the fourth week of pregnancy, neurulation occurs (the formation of a rudimentary central nervous system). This happens through a complex interplay of gene expression and environmental factors (e.g. dietary folic acid) starting with the formation of the neural plate, then the neural groove, and ultimately the neural tube which runs along the embryo’s midline and will give rise to the brain and spinal cord. Some cells from neural folds separate and become neural crest cells that differentiate into the peripheral nervous system. By the sixth week, eyes begin to form and at the end of the eighth week, all organ systems have begun formation. For a view of the stages of human embryonic development see the Multi-Dimensional Human Embryo site.
Determination of pregnancy
One of the first indications that a woman may be pregnant is a missed period or the absence of menstruation. This is not the most reliable sign of pregnancy because there are many reasons for a late or skipped period other than pregnancy. If however there is a combination of factors such as elevated basal body temperature post ovulation, fatigue, nausea, and tender breasts then one may be pregnant. The easiest way to find out is to use a home pregnancy test. These over-the-counter tests indicate the presence of hCG in the urine which can be detected as early as 10 days post-conception. When used properly these tests are highly accurate, however they can produce false-negative or false-positive results. Timing is important and if in doubt confirming the result with a healthcare provider is essential.
Clinicians can look for additional early signs of pregnancy during a pelvic exam such as a softened cervix and color changes in the vagina (more blue than pink). Thick mucus discharge from the vagina may be an indication of the formation of a cervical plug (mucus that blocks the opening of the cervix). An ultrasound can confirm pregnancy as early as five weeks however most physicians will wait until 12 weeks to perform an ultrasound.
Timing of pregnancy
In mammals there is a relationship between the length of time it takes to go from fertilization to delivery (gestation period) and whether or not the neonate is altricial (relatively immature, i.e. usually hairless, and their eyes are often closed) or precocial (relatively developed, i.e.. eyes fully open and operating). For precocial species (e.g. elephants, moose, and primates) gestation periods are “on average approximately four times longer than in altricial species for any given maternal body size” (Martin and MacLarnon 1985). Generally, precocial species give birth to singletons whereas altricial species give birth to multiples in litters. It is hypothesized that the increased length of the gestation period in precocial mammals results in increased development of the central nervous system (Sacher and Staffeldt 1974). However, in humans, the gestation length is approximately nine months which is “shorter than expected based on how much growth the neonatal brain must achieve to reach adult size” perhaps due to the metabolic constraints between the developing fetus and the mother (Dunsworth et al. 2012).
How long a person is pregnant is the actual duration of pregnancy, starting from the date of conception (fertilization) to the time of delivery. This is related to the gestational age which is calculated from the first day of the woman’s last menstrual period (LMP). Although conception usually occurs about two weeks later the LMP approach is used because the exact date of conception is often challenging to determine but the LMP is usually more easily recalled and recorded. The developmental age is the number of weeks since fertilization and thus the gestational age is usually two weeks later than the developmental age. Terminology is often confused but it is important to understand the distinction between an embryo, fetus and infant. The term embryo is used from fertilization to 10 weeks gestational age, fetus from 10 weeks to birth and infant is used from delivery until one year of age.
Pregnancy is divided into three trimesters, each consisting of three calendar months.
- The first trimester: Weeks 1 to 12
- The second trimester: Weeks 13 to 27
- The third trimester: Weeks 28 to 40 (or until birth)
Infants delivered prior to 37 weeks of gestation are considered preterm, while those after 42 weeks are considered post-term.
The risk of multiple complications is much greater for premature infants and it is important to note that in regards to preterm birth incidences “there are significant racial and ethnic disparities. Black race is an established risk factor for spontaneous preterm birth, and preterm birth rates in the United States are 48% higher among non-Hispanic black women compared to women of other racial groups” (Purisch and Gyamfi-Bannerman 2017). In California, a recent study found that the risk of infant mortality was double for African Americans compared to whites (Ratnasiri et al. 2020). This is likely due to differences in access to prenatal care for people of color as well as “racism, and chronic stress” (Hill, Artiga, and Ranji 2022). Post-term pregnancy is also associated with increased problems including neonatal mortality and significant risks to the mother (Galal et al. 2012).
Changes during pregnancy
As stated in the book “Our Bodies, Ourselves” rather than a medical problem, “pregnancy is a natural process… a full-body experience… every organ system adapts” and thus pregnant women experience several physiological changes. There are several common issues associated with pregnancy including back pain, edema, hemorrhoids, pica, round ligament pain and varicose veins.
Physiological changes during pregnancy (Carlin and Alfirevic 2008)
Cardiovascular – cardiac output increases by 30-50% and systemic vascular resistance decreases during pregnancy. This may lead to edema (swollen ankles and fingers).
Pulmonary – There is an increase of 30 to 40% in tidal volume during pregnancy despite the fact that total lung capacity is decreased by 5% due to the elevation of the diaphragm.
Renal – Kidneys increase in size and the ureters dilate during pregnancy. The glomerular filtration rate (GFR) increases by 50% as a result of increased GFR blood urea nitrogen and creatinine decrease by about 25%. Urinary frequency increases as a result of pressure from the enlarging uterus on the bladder.
Hematology – The plasma volume increases by 50%, and the red blood cell volume increases by 20 to 30%, which leads to a decrease in the hematocrit the white blood cell count increases during pregnancy.
Skin – Numerous changes in skin color occur during pregnancy including spider angiomata and hyperpigmentation of nipples thanks to increased estrogen.
GI tract – Elevation in serum progesterone and estrogen levels is responsible for smooth muscle relaxation in the GI tract leading to nausea and vomiting, termed morning sickness even though it can occur anytime during the day. This usually resolves by 14 to 16 weeks gestation. Prolonged gastric emptying times can lead to reflux. Enlarged bowel leads to increased water absorption and constipation.
Musculoskeletal – Hormones cause the ligaments that hold the sacroiliac joints and the pubic symphysis in place begin to soften and stretch. This is accompanied by a shift in the center of gravity which may affect balance and posture.
Cramps can be felt in the 2nd and 3rd trimesters (Braxton Hicks contractions) which are muscle contractions that are irregular and cause discomfort but are harmless and are a way of preparing for true labor (Raines and Cooper, 2017).
Pregnancy complications
During pregnancy, serious health problems may arise. Complications that involve the mother’s health, the fetus’s health, or both need to be addressed and they include the following.
Ectopic Pregnancy – When a fertilized egg grows outside of the uterus (over 90% occur in a fallopian tube). The incidence of ectopic pregnancy is 1% and some resolve spontaneously however it can be life-threatening and requires surgery (Varma and Gupta 2012).
Incompetent cervix – Weakened cervical tissue can lead to premature delivery.
Age-related complications – Advanced maternal age (>35) is associated with a variety of complications such as miscarriage, chromosomal abnormalities, fetal growth restriction, preterm labor, preeclampsia, and gestational diabetes mellitus (Frick 2021).
Placenta accreta – The placenta attaches too deeply into the uterine lining and does not separate easily following delivery. This may cause life-threatening bleeding and/or lead to premature delivery.
Placenta previa – Abnormal implantation of the placenta over the internal cervical os. It can result in premature delivery.
Preeclampsia – Hypertension that usually occurs in the third trimester. Up to 20% of women with preeclampsia have life-threatening complications (Magee, Nicolaides, and von Dadelszen 2022).
Gestational diabetes mellitus (GDM) – Glucose intolerance with onset during pregnancy, is observed in 7% of pregnancies and increases the risk of fetal death during the last 4-8 weeks of gestation (ADA, 2004). Placental overgrowth is associated with GDM.
Uterus didelphys – A woman with two uteri. This is a congenital condition that occurs on rare occasions during embryonic development due to the “complete duplication of uterine horns, cervix, and very often also the vagina…Most women with a uterus didelphys are asymptomatic” (Ćwiertnia et al. 2022). However, the condition does increase the risk of “adverse pregnancy outcomes” such as preterm labor, breech delivery, miscarriage, and decreased live births (Slavchev et al. 2020).
Miscarriage – The “most common serious pregnancy complication is miscarriage” occurring in 11-20% of all clinically recognized pregnancies, the majority due to chromosomal abnormalities (Bottomley and Bourne 2009). A miscarriage is diagnosed usually by vaginal bleeding and the passage of products of conception vaginally.
All pregnancies carry risks however according to the NICHD the designation of “high-risk” pregnancy is for women with the following factors:
- Preexisting health conditions (e.g. diabetic or HIV-positive)
- Overweight
- Multiple births (carrying more than one fetus)
- Age (under 17 or over 35)
Prenatal care
It is very common (>88% in 2021 reported by the CDC) for pregnant women to make regular visits to an OB/GYN (obstetrician/gynecologist) or family physician for prenatal care. Prenatal care includes the following: a routine physical examination (including pelvic examination) at the initial visit, maternal weight and blood pressure at all visits, fetal heart rate auscultation after 10 to 12 weeks with a Doppler monitor or after 20 weeks with a fetoscope, fundal height after 20 weeks, and fetal lie by 36 weeks. Counseling on diet and supplementation is also usually included to reduce the risks of obesity and diabetes and pass along information about folic acid in development. Whether or not prenatal care actually improves outcomes is questionable. Fiscella (1995) found no conclusive evidence to support the need for prenatal care. However, a study by Tandon et al. (2012) found a small reduction in preterm births in women receiving group prenatal care when compared with those receiving traditional care.
Prenatal diagnostic tests
Women with a family history of a genetic disorder or those considered to be in a high-risk pregnancy category may choose prenatal testing to identify potential health problems such as sickle cell anemia or disabilities such as spina bifida. Thanks to technological advancements there are a number of issues that can be diagnosed in different ways including imaging, blood tests, and chromosomal analysis.
Common diagnostic prenatal tests
- Ultrasound, Doppler, and MRI imaging – Real-time visualization of fetal anatomy to identify structural abnormalities through different non-invasive technologies (Reddy et al., 2008).
- Non-invasive prenatal testing (NIPT) – analysis of cell-free DNA (cfDNA) circulating in the mother’s blood which consists of a combination of fetal and maternal components. Used to test for common autosomal aneuploidies and trisomies, sex chromosome aneuploidies, microdeletions, and duplications, and some known monogenic disorders (Kypri et al. 2022). This can be done after 10 weeks of pregnancy however costs are not typically covered by insurance.
- Amniocentesis – Direct sampling of fetal cells from the amniotic fluid around week 16 using a needle inserted through the abdomen. Cells are cultured and provide chromosomal information and neural tube defect information within 10-14 days.
- Chorionic villus sampling (CVS) – Direct sampling of placental tissue is usually done around the 12th week using a needle inserted through the abdomen or vagina. Chromosomal information is available within 10-14 days. Both amniocentesis and CVS carry a very slight risk of pregnancy loss. A report on several studies examining pregnancy loss 14 days after an amniocentesis / CVS showed 0.6% and 0.7% respectively (Mujezinovic and Alfirevic 2007).
Labor and delivery
Labor
Early labor is often marked by persistent contractions which cause the cervix to open (dilate). Dilation is measured in centimeters. How much dilation has occurred allows one to determine how far along in labor one is.
The Bishop score to determine progress
According to Wormer, Buer, and Williford (2022), the Bishop score is used by obstetricians to determine how far along a woman is in labor. This score includes dilation as well as effacement (thinning of the cervix), position, and consistency of the cervix. It also takes into account the position of the fetal head (station) in relation to the ischial spines of the maternal pelvis. A Bishop score of 8 or higher is consistent with spontaneous labor and is used to induce labor. A score of less than 6 is considered unfavorable for induction. Induction is common with over 20% of women in the United States experiencing induced labor. The length of labor varies and is unpredictable however it often shortens with subsequent births.
Obstetric examination to assess fetal lie
It is important to determine if the fetus is presenting in the breech (feet first) or cephalic (head first) position. A noninvasive way to determine the position, presentation, and engagement of the fetus in utero is known as the Leopold maneuvers typically performed by a nurse. The nurse palpates the upper abdomen, on the sides of the uterus, and above the pubic symphysis. Confirmation of the fetal lie can be made with an ultrasound.
The three stages of labor
Stage 1 – onset of labor until dilation and effacement of the cervix are completed
Stage 2 – time of full dilation until delivery
Stage 3 – post delivery of the infant to delivery of the placenta
Vaginal delivery
Iversen, Kahrs, and Eggebo (2021) describe the vaginal delivery process. Once the fetus begins crowning (the head becomes visible through the opening of the vagina) vaginal delivery can begin. This is often accompanied by a burning or stinging sensation. At this point the clinician or midwife will assist in the delivery. To “overcome the resistance met by the maternal bony pelvis and soft tissue” four cardinal movements happen during stage 2 of labor (pictured in the image below from Iverson, Kahrs, and Eggebo, 2021):
- Flexion of the head = the head of the fetus is in a presentation position.
- Internal rotation = the top of the fetal head rotates away from the ischial spines located laterally.
- Extension of the head = the fetal head can pass the maternal pubic symphysis.
- External rotation of the shoulders = the anterior shoulder can be delivered.
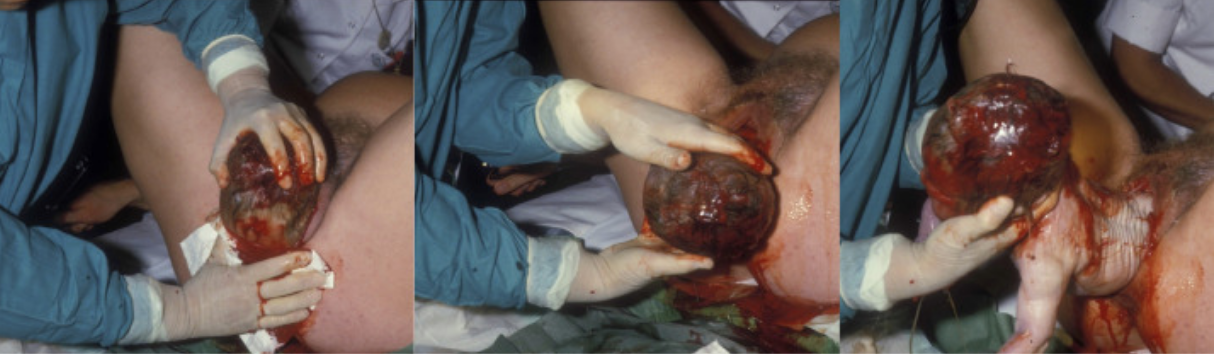
Episiotomy
An episiotomy is a small incision made in the perineum to widen the opening of the vagina. This is done to avoid tearing of the skin and assist in delivery. Stitches are required and should heal within one month after birth.
Assisted vaginal delivery
A healthcare provider may use instruments to assist in the delivery of a baby through the birth canal. It is typically employed when the natural process of labor and childbirth is not progressing as expected, or when there are concerns about the health of the mother or baby. According to O’Mahony, Hofmeyr, and Menon (2010) assisted delivery can involve 1) forceps delivery – forceps are metal instruments curved to position around the infant’s head; and 2) vacuum extraction – a vacuum cup is placed on the fetal scalp and a suction device is connected to the cup to assist.
Cesarean section
When there is a failure to progress in labor cesarean section is used as an emergency measure. Typically a horizontal incision is made in the mother’s abdomen and uterus. Cesarean section may be planned ahead of time under certain circumstances such as: when the mother’s pelvis is too small or the fetus is too large, placenta previa, placental abruption, active herpes lesions, or previous cesarean section. An emergency cesarian section is indicated under the following conditions: fetal distress, breech presentation, shoulder presentation, and cord prolapse. Globally, from 2010-2018, over 20% of women gave birth by cesarian (Betran et al. 2021). Women in higher socioeconomic groups are more likely to choose elective cesarian even though “evidence suggests that cesarian section has a much higher risk than labor” (Singh, Pradeep, and Jauhari 2020).
Pain during labor
Fear and anxiety around the level of pain one may experience increases the chance of having a negative experience during labor and delivery (Hanna-Leena Melender 2002). Under the conditions of typical labor, a woman certainly experiences pain, however, the level of pain varies across individuals. For those electing natural childbirth, the key is preparation regarding the experiences of labor and delivery. Knowing what to expect and learning relaxation and breathing techniques help women deal with the pain. Mental preparation also helps, Duncan et al. (2017) found that preparation with mindfulness training helps women better cope with the pain, and results in greater natural childbirth satisfaction.
Analgesia and anesthesia
Labor is a unique experience for every woman. Depending on the circumstances one may not know until labor begins what approach to deal with pain will be best. There are pain relief options, analgesics reduce pain while anesthesia blocks pain:
- Nitrous Oxide (laughing gas) – inhaled, can be directly controlled by the patient to dull pain and lower anxiety. Effects wear off quickly. For those requiring an episiotomy local infiltration with an anesthetic is used.
- Pudendal block – The pudendal nerve is a sensory and motor nerve arising from the sacral plexus and forms from the ventral spinal nerve roots S2-S4 (back of the pelvis to all the muscles, and skin in the pelvic region). To block the pudendal nerve, an anesthetic is administered either transvaginally or transperineally. It is commonly used in the case of vaginal delivery with either forceps or vacuum.
- Epidural analgesia – The most effective method for reducing labor pain is an epidural which blocks sodium channels in nerve membranes in the lower region of the body (Anim‐Somuah et al. 2018). The anesthetic is administered from a catheter inserted in the spine at the L3-L4 interspace. Some risks of side effects include hypotension, bradycardia, and sedation (van Zuylen et al. 2019).
Using a certified nurse midwife
Midwives are common providers of prenatal care and birth in many countries, however in the United States it is much more common to have a hospital birth attended by an obstetrician-gynecologist. There are different types of midwives, the regulation of which is state-dependent. Certified nurse midwives (CNMs) are registered nurses who must hold a master’s or higher-level nurse-midwivery degree from an accredited program (American College of Nurse Midwives, 2023). In a review conducted by Johantgen et al. (2012) births attended by CNMs compared with those attended by physicians were found to result in better maternal outcomes as well as higher breastfeeding levels. Hamlin et al., (2021) examined outcomes from 136,516 births in military hospitals and found that for both low-risk and high-risk women whose births were attended by CNMs there were improved maternal and natal outcomes. They also had lower instances of having a cesarean birth, induction/augmentation of labor, complications of birth, postpartum hemorrhage, endometritis, and preterm birth.
Planned home birth
It is impossible to predict whether or not everything will go as planned during a birth so most feel that the safest place to give birth is in a hospital. However, most births go smoothly and do not need medical intervention. Moreover, routine medical interventions in hospitals can increase the risk of negative outcomes for mothers and babies (Lothian 2014). Therefore giving birth at home may be a viable alternative. The number of women giving birth at home in the United States has increased significantly over the past few years with a peak of 1.5% observed in January 2021 (CDC, 2022). (Lang 2021) comment that planned home births, also known as community births attended by a registered nurse midwife, have benefits and risks. They recommend that planned home births be limited to low-risk pregnancies which include a single fetus in vertex presentation (head down, head first) with no previous cesarean delivery.
Planned hospital birth is strongly recommended for patients with conditions that increase the risk of maternal or neonatal adverse outcomes. A 5-year long study of Canadian women by Janssen et al. (2009) compared those who planned a hospital birth with a certified nurse midwife in attendance to those who planned a home birth and they found that the maternal outcomes were better for the women who planned a home birth compared to those who planned a physician- attended hospital birth. No significant differences between the two groups were observed for neonatal outcomes. Similarly, Hutton et al.’s (2019) meta-analysis of 500,000 home births, concluded that low-risk women who intend to give birth at home did not exhibit any increase in perinatal and neonatal mortality or morbidity compared to similarly low-risk women who gave birth in a hospital.
Postpartum
The postpartum period lasts approximately six to eight weeks after giving birth. According to the American College of Obstetricians and Gynecologists, this is “ a critical period for a woman and her infant… when she is recovering from childbirth, adjusting to changing hormones, and learning to feed and care for her newborn.”
During the postpartum period, one can expect:
- Contractions, or afterpains, may last for several hours post-delivery.
- Vaginal discharge of the endometrial lining that can last for several weeks.
- Vaginal tear or episiotomy during birth can cause discomfort for a few weeks or longer.
- Incontinence can last for days or weeks due to strain put upon the pelvic floor muscles.
- Hair loss and skin changes due to a drop in progesterone.
- Moodiness and depression due to the sharp decline in progesterone and estrogen.
Postpartum depression
The term clinical postpartum depression is specifically used for depression occurring from birth until one year after. The symptoms are similar to depression such as irritability, anxiety, insomnia, wide mood swings, appetite changes, and hypersensitivity. “Postpartum depression should be distinguished from the postpartum blues, which refers to mood symptoms that are common in the first week to 10 days after delivery and that usually resolve within a few days without any intervention” (O’Hara 2009). According to Patel et al. (2012), the incidence of postpartum depression is reported as up to 15% of all new mothers. Early detection and intervention are important and treatment can be either non-pharmacological or pharmacological.
Breastfeeding
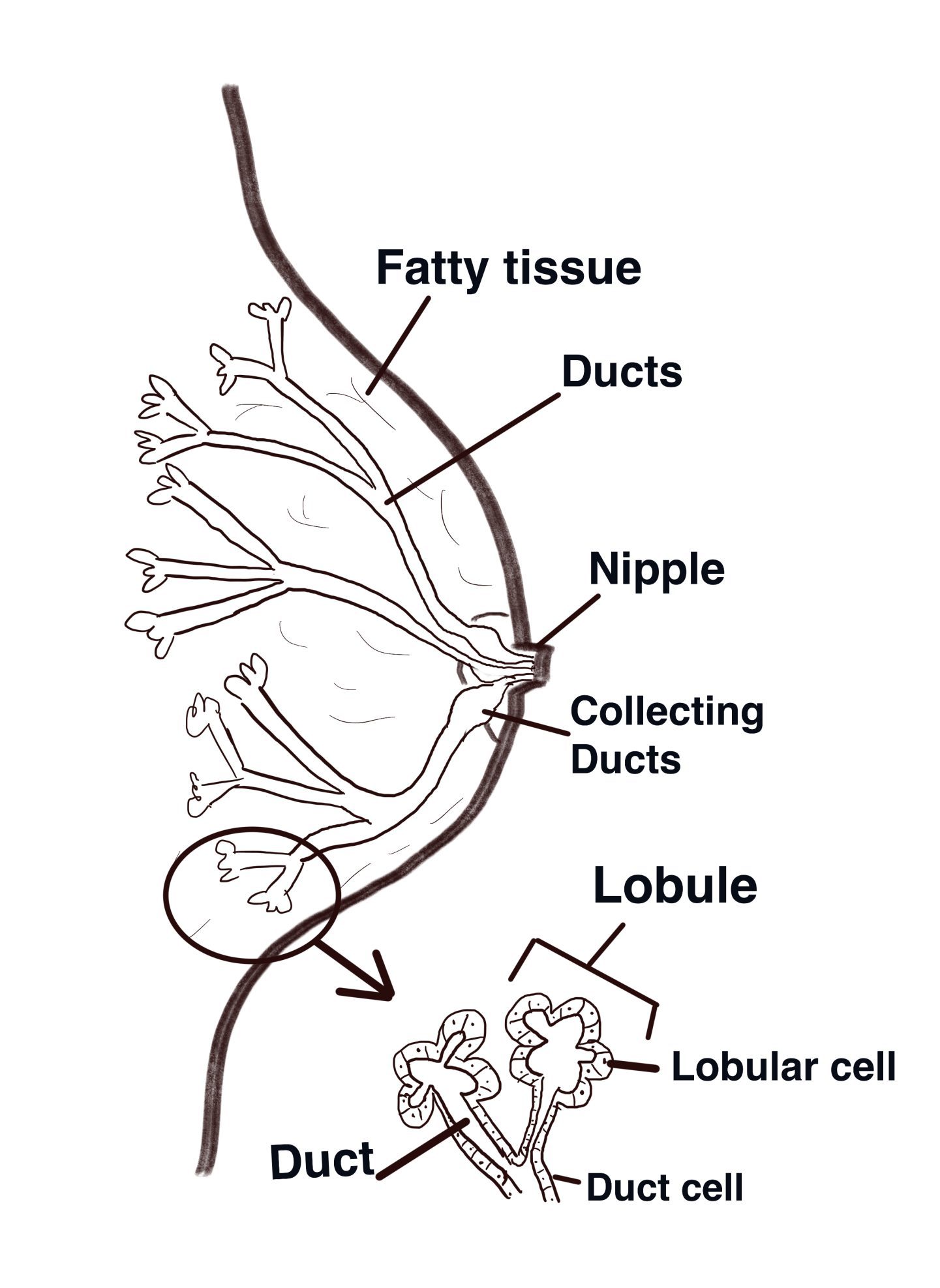
Humans, like all mammals, can provide infants with all the necessary nutrients directly from mammary glands located in the breast overlying the pectoralis major muscles. Mammary glands are present in both females and males but usually are functional only in the female. The adult female breast (illustrated above) contains 15 to 20 lobes of glandular tissue that radiate around the nipple and are supported by connective tissue and adipose (fatty tissue). Each lobe consists of lobules that contain glandular units. A lactiferous duct collects the milk from the lobules within each lobe and carries it to the nipple. Just before the nipple, the lactiferous duct enlarges to form a lactiferous sinus (ampulla), which serves as a reservoir for milk. After the sinus, the duct again narrows and each duct opens independently on the surface of the nipple.
According to the World Health Organization (WHO) early initiation of breastfeeding, within one hour of birth, protects the newborn from acquiring infection and reduces newborn mortality. This is because breast milk is how the “mother’s immunological history can be transferred to her infant, to prime and stimulate the neonate’s immune system as well as provide immediate immunological protection against diseases that the mother has experienced” (Power and Schulkin 2016). Notably, the maturation of the immune system is deferred in early infancy but this is “compensated by immune factors in milk” (Goldman 2002).
Breastfeeding also facilitates the emotional bonding of the mother and the baby and has a positive impact on the duration of exclusive breastfeeding. When a mother initiates breastfeeding within one hour after birth, the production of breast milk is stimulated. The yellow or golden milk produced in the first days, also called colostrum, is an important source of nutrition and immune protection for the newborn. The CDC recommends exclusive breastfeeding for the first 6 months of life, and partial breastfeeding into the second year.
Kent (2007) provides a comprehensive review of breastmilk and breastfeeding
Breast milk is synthesized in the lactocytes that line the alveoli of the mammary gland. Blood capillaries surround the alveoli. Substrates (glucose, amino acids, fatty acids, minerals, and vitamins) diffuse from the blood to the basement membrane of the lactocytes and are used for the synthesis of milk components. These are then secreted across the apical membrane of the lactocyte into the lumen of the alveolus.
There are two stages of milk secretion. Lactogenesis I is the stage in which the mammary glands develop the ability to secrete milk components during the second trimester of pregnancy. Lactogenesis II, which is the onset of copious milk secretion, occurs soon after birth. Lactogenesis II requires adequate levels of prolactin, insulin, and adrenal cortisol, and is triggered by the withdrawal of circulating progesterone after birth.
Each time the baby breastfeeds, oxytocin is released into the circulation. Prolactin plays a role in the control of the amount of milk produced. Measurement of breast volume before and after each breastfeeding for 24 hours has shown that babies will normally drain the breast one or more times each day, but do not drain the breast at each feeding. Because babies feed according to appetite, the mother’s milk production is regulated to match the baby’s milk intake. Current recommendations are to feed babies “on demand”, therefore, there is no prescribed pattern for breastfeeding babies.
The WHO recommends exclusive breastfeeding for the first 6 months of life, and partial breastfeeding into the second year. However, breastfeeding does not always go smoothly and oftentimes women stop within eight weeks (Field et al. 2020). There can be difficulty in an infant latching on, nipples can become wounded and the suckling can be very painful. Feenstra et al. (2018) surveyed over 1,400 mothers who reported that “up to 40% had experienced early breastfeeding problems…including for many mothers…them being in doubt” of whether or not they will be able to provide enough nutrients for their babies through breastfeeding. Disturbing evidence links exposure to forever chemicals, known as PFAS (per- and polyfluoroalkyl substances), to mothers ending breastfeeding prior to six months (Romano et al., 2024).
Evolution of mammary glands
Power and Schulkin (2016) define milk as “a glandular secretion produced by mammalian mothers to feed to their offspring.” All mammalian species (monotremes, marsupials, and eutherians) have milk that is composed of the same ingredients: water, sugars, fats, proteins, vitamins, and minerals. Mammary glands arise from a localized thickening of the epidermis and are made up of epithelial cells organized into alveoli to function as secreting tissue (Boutinaud, Guinard-Flament, and Jammes 2004). In eutherians “lactation is a staggeringly complex phenomenon involving morphological, physiological, biochemical, ecological, and behavioral adaptations” (Farkaš 2015) yet this mechanism appears to have originated long ago. Fossil evidence suggests early mammaliaformes (the last common ancestor to mammals), existing over 200 million years ago, produced a nutrient-rich milk-like secretion (Capuco and Akers 2009). Oftedal (2012) proposes an origin of lactation occurring within the recent mammalian ancestors more than 300 million years ago from an ancestral apocrine structure associated with hair follicles. These are potential precursors to mammary glands because they were likely present in early mammals and primitive apocrine glands “resemble mammary glands in being able to synthesize and secrete lipids and other complex organic molecules.” The apocrine-hair association is observed in monotreme mammary glands presumably providing “moisture and other constituents to permeable eggs” (Farkaš, 2015). Apocrine glands are a type of sweat gland connected to the epithelium and originate from the hair germ (Best and Kamilar, 2018). In mammals, “evidence is consistent with the hypothesis that mammary glands developed from an apocrine-like gland that had an association with hair follicles and sebaceous glands” (Oftedal, 2012).
Mammary glands, an advantage over reptiles?
The ability to produce milk and lactate appears to be a significant adaptation acting first as an “egg-bathing fluid” and ultimately evolving into the “primary source of nutrients for rapidly growing altricial young” (Oftedal, 2002). Two important factors were likely under selection, nutrients provided by milk and protection from the addition of antimicrobial agents within secretions (Capuco and Akers, 2009). Vorbach, Capecchi, and Penninger (2006) argue that lactation primarily “evolved as an inflammatory response to tissue damage and infection.” They cite the existence of two conserved antimicrobial enzymes, Xanthine oxidoreductase (XOR) and lysozyme involved in lactation as being the key reason for the evolution of the mechanism, with additional nutritional roles evolving afterward. XOR plays an integral part in the innate immune system along with lysozyme which comes from a gene that has been duplicated, resulting in ⍺-lactalbumin, an important whey protein found in milk and is involved in lactose synthesis. The ⍺-lactalbumin predates the evolution of mammals, suggesting that “the capacity to produce lactose was an ancient trait that preceded its utility in milk synthesis” (Capuco and Akers, 2009). Whatever the original selective force, clearly the adaptation of lactation allowed our ancestors to “escape the Permian-Triassic extinction…at the end of the Cretaceous” finally becoming, arguably, the dominant land vertebrates of today” (Power and Schulkin, 2016).
Think, Pair, Share
Capacitation is referred to as a “defrosting” process for sperm. Is that an apt term and why do you think this process happens?
Embryo, fetus, and infant are not interchangeable terms. Why are they often confused?
Discuss why determining whether or not one is pregnant is so important during the first five weeks of pregnancy.
Under what circumstances might a woman want to have prenatal testing done?
What arguments can be made in support of the evolution of mammary glands as being the result of a combination of factors?
Deeper thoughts
A woman can reliably determine whether or not she is pregnant using an over-the-counter test and pregnancy can be confirmed approximately three days after a missed period however almost all physicians make patients wait until 12 weeks to perform an ultrasound. What might be the reasoning for this?
What reasons might there be for the high infant and maternal mortality rates still experienced in the United States?
What might societal pressures have to do with prenatal care and whether or not a woman chooses to use anesthesia during labor?
Breastfeeding can be difficult. How might the pressure to breastfeed be connected to postpartum depression and what might be done to alleviate such pressure?
Key Terms
Acrosomal reaction
Allantois
Amniocentesis
Amnion
Amniotic fluid
Blastocyst
Capacitation
Cesarian section
Chorion
Chorionic villi biopsy
Colostrum
Cytotrophoblast
Ectoderm
Ectopic pregnancy
Egg activation
Embryo
Endoderm
Endometrium
Episiotomy
Fallopian tube
Fetus
Gastrula
Gastrulation
Gestational diabetes
Human chorionic gonadotropin
Implantation
Inner cell mass
Intrauterine insemination
Mammary gland
Mesoderm
Miscarriage
Morphogenic determinants
Morula
Neonatal
Oviduct
Placenta
Placenta previa
Postpartum depression
Preeclampsia
Preterm
Prostaglandin
Polyspermy
Syncytiotrophoblast
Trophoblast
Yolk sac
Zona pellucida
Zygote
References
Ana P. B., J. Ye, A. Moller, J. P. Souza, and J. Zhang. 2021. “Trends and Projections of Caesarean Section Rates: Global and Regional Estimates.” BMJ Global Health 6 (6): e005671. https://doi.org/10.1136/bmjgh-2021-005671.
Anim‐Somuah, M., R. M. Smyth, A. M. Cyna, and A. Cuthbert. 2018. “Epidural versus Non‐epidural or No Analgesia for Pain Management in Labour.” 5.
Boitrelle, F., R. Shah, R. Saleh, R. Henkel, H. Kandil, E. Chung, P. Vogiatzi, A. Zini, M. Arafa, and A. Agarwal. 2021. “The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis.” Life (Basel, Switzerland) 11 (12). https://doi.org/10.3390/life11121368.
Bottomley, C., and T. Bourne. 2009. “Diagnosing Miscarriage.” Acute Gynaecology Volume 1: Early Pregnancy Complications 23 (4): 463–77. https://doi.org/10.1016/j.bpobgyn.2009.02.004.
Boutinaud, M., J. Guinard-Flament, and H. Jammes. 2004. “The Number and Activity of Mammary Epithelial Cells, Determining Factors for Milk Production.” Reproduction Nutrition Development 44: 499–508. https://doi.org/DOI: 10.1051/rnd:2004054.
Capuco, A. V., and R. M. Akers. 2009. “The Origin and Evolution of Lactation.” Journal of Biology 8 (4): 37. https://doi.org/10.1186/jbiol139.
Carlin, A., and Z. Alfirevic. 2008. “Physiological Changes of Pregnancy and Monitoring.” Critical Care in Obstetrics 22 (5): 801–23. https://doi.org/10.1016/j.bpobgyn.2008.06.005.
Christians, E., A. A. Davis, S. D. Thomas, and I. J. Benjamin. 2000. “Maternal Effect of Hsf1 on Reproductive Success” 407: 693–94.
Ćwiertnia, A., D. Borzyszkowska, A. Golara, N. Tuczyńska, M. Kozłowski, S. Kwiatkowski, and A. Cymbaluk-Płoska. 2022. The impact of uterus didelphys on fertility and pregnancy. International journal of environmental research and public health, 19(17), p.10571.
Duncan, L. G., M. A. Cohn, M. T. Chao, J. G. Cook, J. Riccobono, and N. Bardacke. 2017. “Benefits of Preparing for Childbirth with Mindfulness Training: A Randomized Controlled Trial with Active Comparison.” BMC Pregnancy and Childbirth 17 (1): 140. https://doi.org/10.1186/s12884-017-1319-3.
Dunsworth, H. M., A. G. Warrener, Deacon, and H. Pontzer. 2012. “Metabolic Hypothesis for Human Altriciality.” PNAS 109 (38): 15212–16.
Evans, J. P. 2020. “Preventing Polyspermy in Mammalian Eggs—Contributions of the Membrane Block and Other Mechanisms.” Molecular Reproduction and Development 87 (3): 341–49. https://doi.org/10.1002/mrd.23331.
Farkaš, R.. 2015. “Apocrine Secretion: New Insights into an Old Phenomenon.” Biochimica et Biophysica Acta (BBA) – General Subjects 1850 (9): 1740–50. https://doi.org/10.1016/j.bbagen.2015.05.003.
Feenstra, M. M., M. J. Kirkeby, M. Thygesen, D. B. Danbjørg, and H. Kronborg. 2018. “Early Breastfeeding Problems: A Mixed Method Study of Mothers’ Experiences.” Sexual & Reproductive Healthcare 16 (June): 167–74. https://doi.org/10.1016/j.srhc.2018.04.003.
Field, E., S. Anipindi, C. Neville, and A. Field. 2020. “Common Breastfeeding Problems.” InnovAiT: Education and Inspiration for General Practice 13: 436–43.
Fiscella, K.. 1995. “Does Prenatal Care Improve Birth Outcomes? A Critical Review.” Obstetrics & Gynecology 85 (3): 468–79. https://doi.org/10.1016/0029-7844(94)00408-6.
Frick, A. P. 2021. “Advanced Maternal Age and Adverse Pregnancy Outcomes.” Reproduction at an Advanced Maternal Age 70 (January): 92–100. https://doi.org/10.1016/j.bpobgyn.2020.07.005.
Galal, M., I. Symonds, H. Murray, F. Petraglia, and R. Smith. 2012. “Postterm Pregnancy.” Facts, Views & Vision in ObGyn 4 (3): 175–87.
Goldman, A. S. 2002. “Evolution of the Mammary Gland Defense System and the Ontogeny of the Immune System.” Journal of Mammary Gland Biology and Neoplasia 7 (3): 277–89. https://doi.org/10.1023/A:1022852700266.
Hanna, C. W. 2020. “Placental Imprinting: Emerging Mechanisms and Functions.” PLoS Genetics 16 (4): e1008709. https://doi.org/10.1371/journal.pgen.1008709.
Hanna-Leena M., R. 2002. “Experiences of Fears Associated with Pregnancy and Childbirth: A Study of 329 Pregnant Women.” 29 (2): 101–11.
Hertwig, O.. 1876. “Beitrage zur Kenntniss der Bildung, Befruchtung und Theilung des thierischen Eies.” 1: 347–432.
Higdon, H. L., D. A. Forstein, A. C. Collins, and W. R. Boone. 2008. “Minimum Number of Sperm Needed to Obtain an Intrauterine Insemination Pregnancy.” In Sperm Preparation. https://doi.org/10.1016/j.fertnstert.2008.07.641.
Hill, L., S. Artiga, and U. Ranji. 2022. “Racial Disparities in Maternal and Infant Health: Current Status and Efforts to Address Them.” Kaiser Family Foundation. 2022. https://www.kff.org/racial-equity-and-health-policy/issue-brief/racial-disparities-in-maternal-and-infant-health-current-status-and-efforts-to-address-them/#:~:text=Research%20has%20documented%20that%20social,of%20mortality%20among%20Black%20infants.
Hutton, E. K., A. Reitsma, J. Simioni, G. Brunton, and K. Kaufman. 2019. “Perinatal or Neonatal Mortality among Women Who Intend at the Onset of Labour to Give Birth at Home Compared to Women of Low Obstetrical Risk Who Intend to Give Birth in Hospital: A Systematic Review and Meta-Analyses.” EClinicalMedicine 14: 59–70. https://doi.org/10.1016/j.eclinm.2019.07.005.
Iversen, J. K., B. H. Kahrs, and T. M. Eggebo. 2021. “There Are 4, Not 7, Cardinal Movements in Labor” 3 (6). https://doi.org/10.1016/j.ajogmf.2021.100436.
Johantgen, M., L. Fountain, G. Zangaro, R. Newhouse, J. Stanik-Hutt, and K. White. 2012. “Comparison of Labor and Delivery Care Provided by Certified Nurse-Midwives and Physicians: A Systematic Review, 1990 to 2008.” Women’s Health Issues 22 (1): e73–81. https://doi.org/10.1016/j.whi.2011.06.005.
Kent, J. C. 2007. “How Breastfeeding Works.” Special Continuing Education Issue 52 (6): 564–70. https://doi.org/10.1016/j.jmwh.2007.04.007.
Kim, S., and J. Kim. 2017. “A Review of Mechanisms of Implantation.” Development & Reproduction 21 (4): 351–59. https://doi.org/10.12717/DR.2017.21.4.351.
Kypri, E., M. Ioannides, A. Achilleos, G. Koumbaris, P. Patsalis, and M. Stumm. 2022. “Non-Invasive Prenatal Screening Tests – Update 2022,” Journal of Laboratory Medicine, 46 (4): 311–20. https://doi.org/10.1515/labmed-2022-0023.
Lang, G. 2021. “Out-of-Hospital Birth.” American Family Physician 103 (11): 672–79.
Lothian, J. A. 2014. “Healthy Birth Practice #4: Avoid Interventions Unless They Are Medically Necessary.” The Journal of Perinatal Education 23 (4): 198–206. https://doi.org/10.1891/1058-1243.23.4.198.
Magee, L. A., K. H. Nicolaides, and P. von Dadelszen. 2022. “Preeclampsia.” New England Journal of Medicine 386 (19): 1817–32. https://doi.org/10.1056/NEJMra2109523.
Martin, R. D., and A. M. MacLarnon. 1985. “Gestation Period, Neonatal Size and Maternal Investment in Placental Mammals” 313: 220–23.
Moore, J. E. 2016. “Women’s Voices in Maternity Care: The Triad of Shared Decision Making, Informed Consent, and Evidence-Based Practices.” 30 (3): 218–23.
Mujezinovic, F., and Z. Alfirevic. 2007. “Procedure-Related Complications of Amniocentesis and Chorionic Villous Sampling: A Systematic Review.” Obstetrics & Gynecology 110 (3). https://journals.lww.com/greenjournal/fulltext/2007/09000/procedure_related_complications_of_amniocentesis.24.aspx.
Nüsslein-Volhard, C., M. Lohs-Schardin, K. Sander, and C. Cremer. 1980. “A Dorso-Ventral Shift of Embryonic Primordia in a New Maternal-Effect Mutant of Drosophila.” Nature 283 (5746): 474–76. https://doi.org/10.1038/283474a0.
OECD. 2023. “Maternal Mortality Rates Worldwide in 2020, by Country.” 2023. https://www.statista.com/statistics/1240400/maternal-mortality-rates-worldwide-by-country/.
Oftedal, O. T. 2012. “The Evolution of Milk Secretion and Its Ancient Origins.” Animal 6 (3): 355–68. https://doi.org/10.1017/S1751731111001935.
O’Hara, M. W. 2009. “Postpartum Depression: What We Know.” Journal of Clinical Psychology 65 (12): 1258–69. https://doi.org/10.1002/jclp.20644.
O’Mahony, F., G. J. Hofmeyr, and V. Menon. 2010. “Choice of Instruments for Assisted Vaginal Delivery.” Cochrane Database of Systematic Reviews, no. 11. https://doi.org/10.1002/14651858.CD005455.pub2.
Patel, M., R. K. Bailey, S. Jabeen, S. Ali, N. C. Barker, and K. Osiezagha. 2012. “Postpartum Depression: A Review.” Journal of Health Care for the Poor and Underserved 23 (2): 534–42. https://doi.org/10.1353/hpu.2012.0037.
Patricia A. Janssen, Lee Saxell, Lesley A. Page, Michael C. Klein, Robert M. Liston, and Shoo K. Lee. 2009. “Outcomes of Planned Home Birth with Registered Midwife versus Planned Hospital Birth with Midwife or Physician.” Canadian Medical Association Journal 181 (6–7): 377. https://doi.org/10.1503/cmaj.081869.
Power, M. L., and J. Schulkin. 2016. Milk: The Biology of Lactation. JHU Press.
Purisch, S. E., and C.Gyamfi-Bannerman. 2017. “Epidemiology of Preterm Birth.” Current Preterm Birth Prevention Strategies 41 (7): 387–91. https://doi.org/10.1053/j.semperi.2017.07.009.
Ratnasiri, A., S. Lakshminrusimha, R. Dieckmann, H. Lee, J. Gould, S. Parry, V. Arief, I. DeLacy, R. DiLibero, and K. Basford. 2020. “Maternal and Infant Predictors of Infant Mortality in California, 2007–2015.” https://doi.org/10.1371/journal.pone.0236877.
Reardon, S.. 2015. “NIH Invests US$41.5 Million in Placenta Research.” Nature, February. https://doi.org/10.1038/nature.2015.17017.
Roberts, R. M., J. A. Green, and L. C. Schulz. 2016. “The Evolution of the Placenta.” Reproduction 152 (5): R179–89. https://doi.org/10.1530/REP-16-0325.
Romano, M. E., L.G. Gallagher, G. Price, K. A. Crawford, R. Criswell, E. Baker, J. Cook Botelho, A. M. Calafat, and M. R. Karagas. 2024. “Plasma per-and polyfluoroalkyl substance mixtures during pregnancy and duration of breastfeeding in the New Hampshire birth cohort study.” International Journal of Hygiene and Environmental Health 258: 114359.
Rossant, J., and J. C. Cross. 2001. “Placental Development: Lessons from Mouse Mutants.” Nature Reviews Genetics 2 (7): 538–48. https://doi.org/10.1038/35080570.
Sacher, G. A., and E. F. Staffeldt. 1974. “Relation of Gestation Time to Brain Weight for Placental Mammals: Implications for the Theory of Vertebrate Growth.” American Naturalist 108: 595–615.
Singh, N., Y. Pradeep, and S. Jauhari. 2020. “Indications and Determinants of Cesarean Section: A Cross-Sectional Study.” International Journal of Applied & Basic Medical Research 10 (4): 280–85. https://doi.org/10.4103/ijabmr.IJABMR_3_20.
Slavchev, S., S. Kostov, and A. Yordanov. 2020. “Pregnancy and childbirth in uterus didelphys: a report of three cases.” Medicina 56(4):198.
Sun, F., A. Bahat, A. Gakamsky, E. Girsh, N. Katz, L. C. Giojalas, I. Tur-Kaspa, and M. Eisenbach. 2005. “Human Sperm Chemotaxis: Both the Oocyte and Its Surrounding Cumulus Cells Secrete Sperm Chemoattractants.” Human Reproduction 20 (3): 761–67. https://doi.org/10.1093/humrep/deh657.
Tam, P. P. L, and R. R. Behringer. 1997. “Mouse Gastrulation: The Formation of a Mammalian Body Plan.” Mechanisms of Development 68 (1): 3–25. https://doi.org/10.1016/S0925-4773(97)00123-8.
Tandon, S. D., S. Darius, L. Colon, P. Vega, J. Murphy, and A. Alonso. 2012. “Birth Outcomes Associated with Receipt of Group Prenatal Care among Low‐income Hispanic Women” 57 (5): 476–81.
Tyser, R. C. V., E. Mahammadov, S. Nakanoh, L. Vallier, A. Scialdone, and S. Srinivas. 2021. “Single-Cell Transcriptomic Characterization of a Gastrulating Human Embryo.” Nature 600 (7888): 285–89. https://doi.org/10.1038/s41586-021-04158-y.
Van Gronigen C. G., K. M. Storey, L. E. Parmeley, and L. C. Schulz. 2021. “Effects of Maternal Nutrient Restriction during the Periconceptional Period on Placental Development in the Mouse.” PLoS ONE 16 (1): e0244971.
van Zuylen, M. L., W. T. Hoope, E. Bos, J. Hermanides, M. F. Stevens, and M. W. Hollmann. 2019. “Safety of Epidural Drugs: A Narrative Review.” Expert Opinion on Drug Safety 18 (7): 591–601. https://doi.org/10.1080/14740338.2019.1617271.
Varma, R., and J. Gupta. 2012. “Tubal Ectopic Pregnancy.” BMJ Clinical Evidence 2012 (February): 1406.
Vorbach, C., M. R. Capecchi, and J. M. Penninger. 2006. “Evolution of the Mammary Gland from the Innate Immune System?” BioEssays 28: 606–16.
Wormer, K. C., A. Buer, and A. E. Williford. 2022. “Bishop Score.” StatPearls. 2022. https://www.ncbi.nlm.nih.gov/books/NBK470368/.
Suggested Reading
“What to Expect When You’re Expecting” by Heidi Murkoff and Sharon Mazel – This classic pregnancy guide offers week-by-week information on fetal development, changes in the mother’s body, and practical advice for a healthy pregnancy.
“Mayo Clinic Guide to a Healthy Pregnancy” by the Mayo Clinic – Written by a team of healthcare experts, this comprehensive guide covers every aspect of pregnancy, from conception to childbirth, and provides evidence-based advice.
“The Expectant Father: The Ultimate Guide for Dads-to-Be” by Armin A. Brott and Jennifer Ash – Geared towards expectant fathers, this book offers insights into the emotional and practical aspects of pregnancy and fatherhood.
“Ina May’s Guide to Childbirth” by Ina May Gaskin – Midwife Ina May Gaskin shares her extensive experience in natural childbirth, offering a reassuring and informative perspective on labor and delivery.
“The Birth Partner: A Complete Guide to Childbirth for Dads, Partners, Doulas, and All Other Labor Companions” by Penny Simkin – This book is a valuable resource for birth partners and support persons, providing practical advice on how to assist and comfort the laboring mother.
“The Pregnancy Book: Month-by-Month, Everything You Need to Know from America’s Baby Experts” by William Sears, M.D., and Martha Sears, R.N. – The Sears family, known for their attachment parenting philosophy, provides a month-by-month guide to pregnancy, focusing on a holistic approach to prenatal care.
Interesting Podcast
Is it normal? The Pregnancy Podcast with Jesse Ware Breastfeeding
