5 Reproductive Cycle
STUDENT LEARNING OUTCOMES
At the end of this chapter you should be able to:
- Explain the onset of puberty
- Describe how the HPG axis regulates the reproductive cycle
- Recall how the dominant follicle is recruited and ruptures
- Summarize the phases of the ovarian and menstrual cycle
- Illustrate the changes in hormone levels associated with the ovarian and menstrual cycles
- Predict how an increase/decrease in progesterone impacts the endometrial lining
- Compare and contrast estrus with menses
Historically, menstrual health has received little attention

As an undergraduate student at Wellesley College, biopsychologist Martha Kent McClintock examined how menstrual cycles become synced when women live close together. As Hermes (2022) contends, McClintock went on to write “what is likely the most famous honors thesis in history, which was ultimately published in Nature in 1971” and then to study the effect of pheromones on the cycle, showing how ovarian-dependent pheromones can regulate ovulation in women within a social group (Stern and McClintock 1998). In an interview by Natalie Angier in the New York Times (1998), McClintock postulated that the ability to pick up social cues and incorporate them into her cycle is beneficial because ”reproduction is a very time-consuming, risky business, and the more cues a female has to her social setting, and whether this is the right time to try getting pregnant, the better off she’ll be.” McClintock’s pioneering work provided insight into the complexity of women’s reproductive cycles which are not only regulated by multiple endogenous controls (having an internal cause, e.g. hormones) but also impacted by exogenous factors (having an external cause, e.g. stress). However, a gap in our knowledge of women’s reproductive cycle, specifically uterine and menstrual physiology, persists due to technical, scientific, and societal barriers (Critchley et al. 2020).
Intro to puberty and an overview of the hypothalamus-pituitary-gonadal axis (HPG)
All oocytes (female germ cells) for an individual mammalian female are produced before birth during embryogenesis. Recall from the Genetics of Sex chapter that the primordial germ cells arise early in development and migrate to the embryo’s genital ridge. Duncan et al. (2020) review how the germ cells divide mitotically to increase in number and transform into oogonia as the differentiation into the female gonads occurs. After multiple mitoses, the oogonia become primary oocytes that enter meiosis, Prophase I, and remain dormant until puberty. Upon puberty, each month a subset of ovarian follicles mature and MII will resume at fertilization (see illustration below).
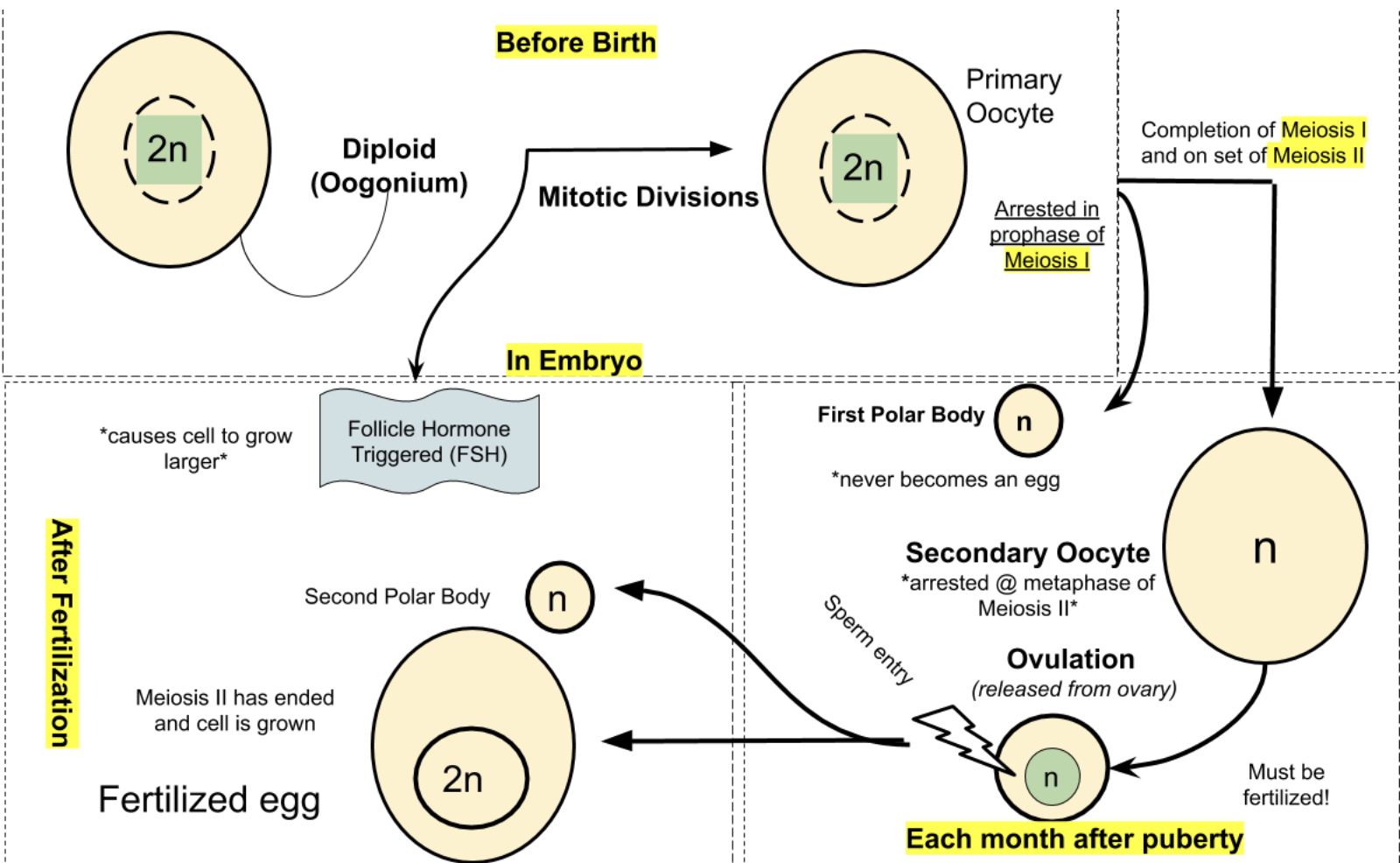
Puberty is brought on by a complex series of events involving the endocrine system that concludes with sexual maturity. The endocrine system utilizes hormones to regulate and coordinate a wide range of physiological processes within the body. Hormones are chemical messengers produced by specialized glands and tissues, and they play a vital role in the development and maintenance of the reproductive cycle. This requires an intricate messaging system involving the brain and ovaries referred to as the hypothalamic -pituitary-gonadal (HPG) axis. It also involves the production of adrenal androgens which are hormones secreted by the adrenal cortex, the outer layer of the adrenal glands, situated on top of each kidney. Adrenal androgens including dehydroepiandrosterone (DHEA) and androstenedione contribute to the development of secondary sexual characteristics such as the growth of pubic and axillary (underarm) hair.
The HPG
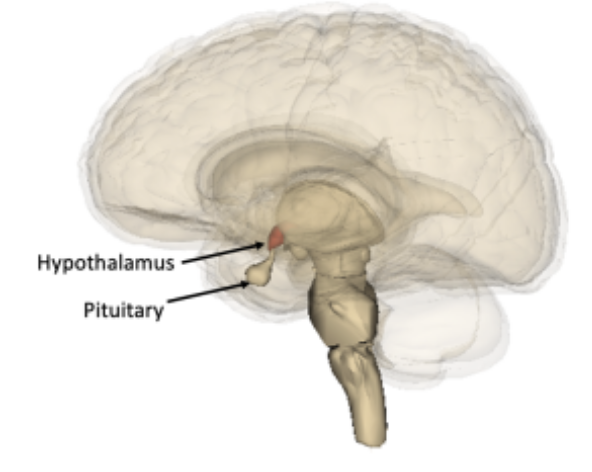
At the base of the brain, under the thalamus, lies the pea-sized hypothalamus. The hypothalamus is highly specialized and coordinates the endocrine system. The hypothalamus functions in association with the pituitary gland to which it is attached (see image). The hypothalamus receives signals and has neurons that release hormones that travel to the anterior pituitary and trigger the release of other gonadotropins (hormones that regulate the activity of the gonads).
The onset of puberty is marked by a reactivation of the HPG, which is suppressed during early childhood. Among the various hormones produced by the hypothalamus, gonadotropin-releasing hormone (GnRH) is specifically involved in initiating the reproductive cycle. The onset of puberty occurs when “signals from the central nervous system and signals from the periphery indicate systemic health and adequate nutritional status converge in the hypothalamus to stimulate GnRH pulsatile secretion” (DiVall and Radovick 2008). GnRH binds to receptors in the pituitary gland stimulating the release of gonadotropins.
The age of onset varies widely among young girls ranging from 8-14 years of age. Both genetics and environmental factors are thought to impact the timing of pubertal onset (Uenoyama et al. 2019). Rubin et al. (2009) annually surveyed over 4,600 girls over a six-year timeframe (ages 8-12) and found a strong relationship between a mother’s and daughter’s age at menarche (the first period) suggesting a potential genetic factor. Another key determinant appears to be the amount of gluteofemoral fat a young woman has (Lassik and Gaulin 2007). This is because a certain threshold of fat cells is needed to produce enough estrogen to activate puberty and having sufficient body fat stores as the trigger for the reproductive cycle would be an adaptation “for ensuring that pregnancy will not occur unless there are adequate fat stores to sustain both the mother and the growing fetus” (Kaplowitz 2008). However different studies on the link between body fat and menarche provide conflicting results (Walvoord 2010).
Pulsating GnRH and the onset of puberty
The preoptic area (POA) of the hypothalamus houses GnRH-neurons that release GnRH in “a pulsatile pattern that in turn stimulates the pulsatile release of two gonadotropins from the anterior pituitary: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The activation of the gonadotropins is completed by various signaling pathways dependent on the GnRH pulse frequency (Shalev and Melamed 2020). The onset of pubertal maturation occurs thanks to a steady rhythmic increase in LH and the progression of puberty continues as the LH amount released increases (DiVall and Radovick 2008).
As puberty progresses in humans the ovaries become receptive to the gonadotropins and begin producing estradiol (a form of estrogen). Estradiol is the key hormone responsible for the development of breasts, and growth spurt. Accompanying the ovarian production of estradiol, the adrenal glands secrete androgens that cause pubic and axillary hair development. Physicians use what is referred to as Tanner staging to determine how individuals are progressing through puberty. This is based on clinical evaluation of pubic hair growth and breast development and provides a simple, ordinal scale that ranges from 1 (prepubertal) to 5 (postpubertal) (Tanner and Whitehouse 1976). Menarche, the first period, occurs “after a yearlong rise in daily estradiol output” (DiVall and Radovick, 2008).
An overview of the ovarian cycle and the menstrual cycle
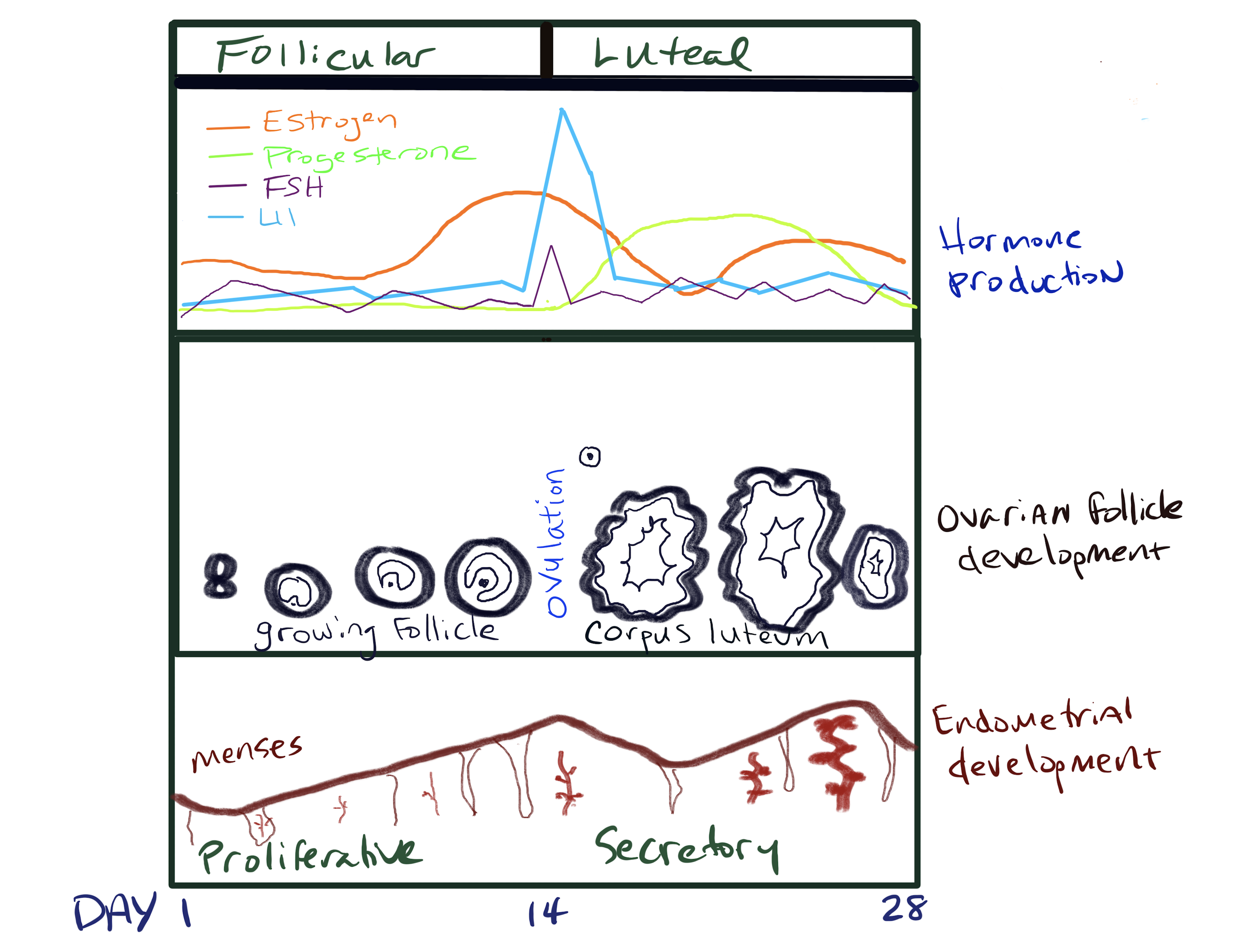
Joanne Richards has been studying the follicular ovarian cycle for over 40 years. Richards (2018) aptly describes the ovarian cycle as “an exquisite, highly orchestrated, and complex developmental process that depends on functional interactions and critical signaling pathways…”. It is characterized by a cyclic pattern of hormonal changes regulated by feedback mechanisms. The ovarian hormones, estradiol, and progesterone are the main modulators of the hypothalamic–pituitary axis. The ovarian cycle is interconnected with the menstrual cycle which is also influenced by the ovarian hormones. Once the ovary is “awakened” during puberty and begins producing estradiol, the process of selection and release of primordial follicles occurs. In humans, the 28-day ovarian cycle is governed by hormones but bear in mind that there is a great deal of variability in cycle length (Rees 1997).
The ovarian cycle has two phases: the follicular and luteal phases. The follicular phase results in the maturation of follicles and ovulation, and the luteal phase involves the development of the corpus luteum (an endocrine structure within the ovary that produces hormones to maintain a uterine environment for potential pregnancy). Concurrently, the menstrual cycle, also controlled by hormones, is responsible for the degeneration and reconstruction of the uterine lining for the successful implantation and support of the embryo. The menstrual cycle is divided into three phases: menses, the proliferative phase, and the secretory phase. Menstruation is exhibited in primate mammals as well as a few species of bats and shrews.
Q&A with Dr. Joanne Richards, Ph.D.

Professor in the Department of Molecular and Cellular Biology (MCB) and the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston, Texas.
What drew you to becoming a scientist? My life in science came as a surprise, a discovery! It was not a childhood dream; it was not planned. Even today, I do not think of myself as a ‘scientist’. What I enjoy and what has captured me is the process of discovery and the challenges of deciphering the secrets of Mother Nature. Most rewarding have been the unexpected results that have led me down new paths and reminded me that one should always keep an open mind!
What do you like most about your career? Research is an exacting but exciting career. It is the discovery mode of science and research that is the most fun. It is a detective game! Research in the ovary is particularly intriguing because one studies how ovarian oocytes (eggs) are contained in follicles that are stimulated to grow by hormones coming from the pituitary, FSH, and LH.
How do you see the career path for women in science has changed since you began and what do you hope for the future? There are many more women in science today than when I received my Ph.D. And I think women are contributing significantly in many areas of scientific research. One has only to look at recent women in medicine, biology, and chemistry who have received a Nobel Prize. At scientific meetings, women are no longer a minority but a major force, presidents of societies, panel members on NIH grant reviews, etc. I believe that pattern will continue. Young women just need to decide if science is what they want.
What advice do you have for future women scientists? If you like to discover how things work, you will enjoy a career in science. Scientists in general are not researching to make money. Scientists are trying to understand how things work, to come up with better ways to cure diseases, to identify new compounds to cure diseases, and to identify the basis of diseases to improve human health, prevent diseases, control climate damage, etc. Scientific research is not easy. One has to learn that if an experiment does not work, there is a reason. And if it is not a technical issue then there must be something important to explore. One has to be able to persevere! And to like challenges! But the rewards are realizing what you can and do contribute.
The ovaries and ovarian follicles
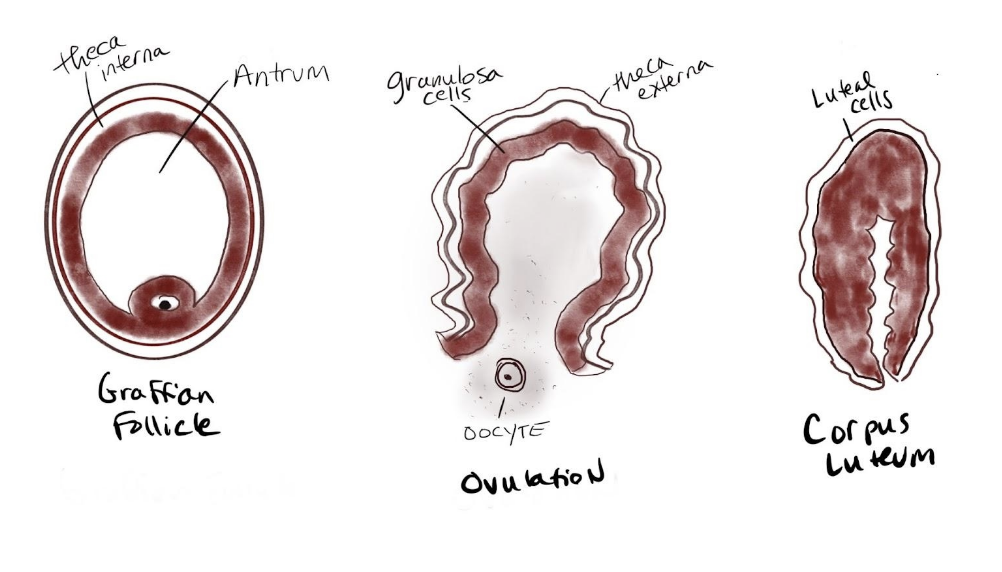
The ovaries are almond-sized (approximately 30 mm long, 25 mm wide, and 15 mm on average) and covered by a thick epithelium. The tissue of the ovaries is divided into two distinct regions: an inner medulla and an outer cortex. The medulla is a loose connective tissue that contains blood vessels, lymph tissue, and nerve cells. Within the cortex reside the ovarian follicles (in different developmental stages, see image below). According to Duncan et al. (2020) follicles are differentiated in size due to differences in their growth phase based on level of maturity: primordial follicles are prenatal, antral follicles are those where a noticeable cavity filled with follicular fluid has developed and Graaffian follicles are mature with the oocyte that is ready to be ovulated. Follicles contain the oocyte and provide an environment for the accumulation of nutrients for future embryogenesis and growth. The oocyte is surrounded by two cell types that work together to produce estrogen: theca cells and granulosa cells.
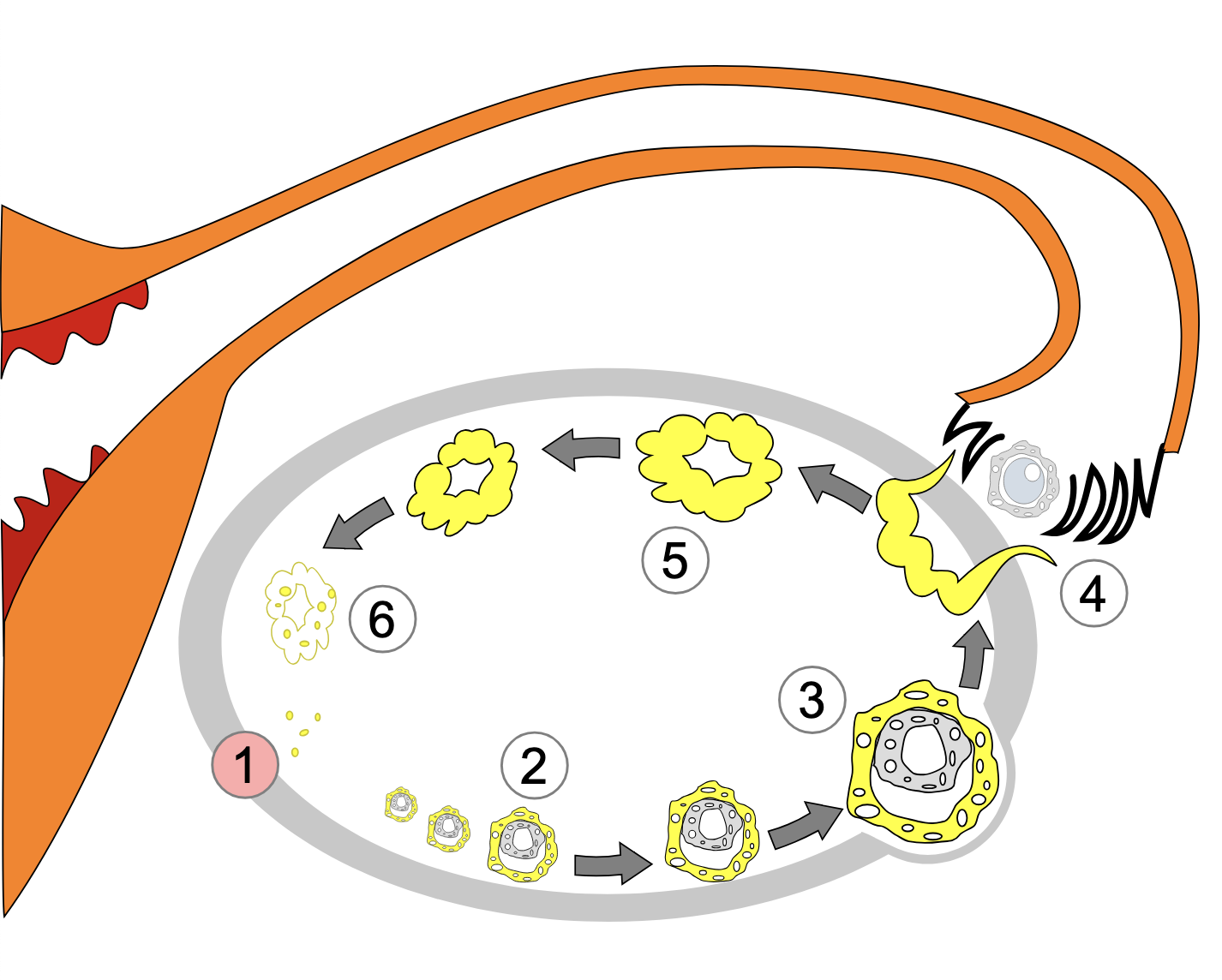
Recruitment and differentiation of theca cells
Each mammalian female has an “ovarian reserve” of primordial follicles, most of which degenerate through a process known as atresia. However, a select few are recruited to complete the final growth phase and get ovulated on a cyclical basis throughout their reproductive lives (see diagram below, modified from Hsueh et al., 2015). By the 20th week of human embryonic development, the number of initial primordial follicles in the fetus is approximately 6 to 7 million. By puberty, the number has been reduced to approximately 400,000-500,000 follicles (Oktem and Urman 2010). During reproductive life, the continuing growth of primordial and primary follicles into secondary and larger follicles leads to a gradual decrease in the original follicle pool. McGee and Hsueh (2000) provide a detailed description of the recruitment process. From early embryonic development until puberty, some of the primordial follicles become stimulated to initiate growth, whereas the rest of the follicles remain quiescent for months or years. The initially recruited primordial oocytes remain arrested in prophase I of meiosis. The remaining follicles not yet recruited, remain dormant and are probably under some type of constant inhibitory influences to remain that way. Once puberty begins, cyclic recruitment occurs whereby a cohort of follicles is “rescued” from atresia known as antral follicles. From the cohort, a single follicle becomes the dominant follicle as depicted in the diagram below adapted from Hsueh et al (2015).
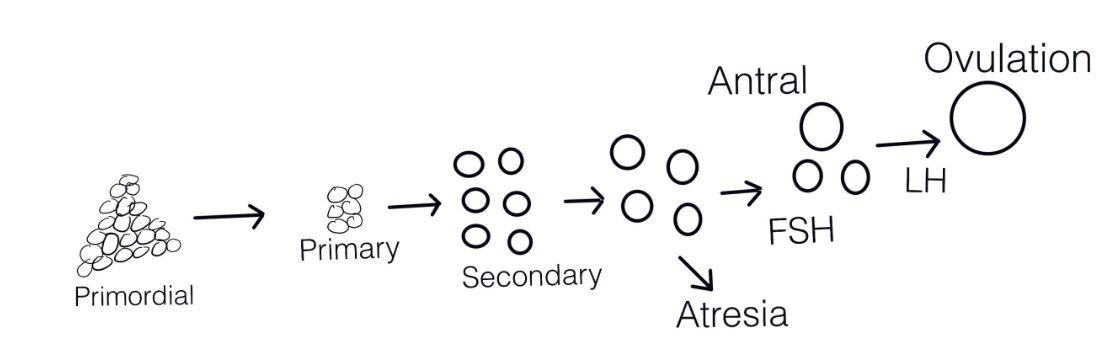
The ovaries experience cyclic changes that involve the maturation and release of eggs as well as the production of progesterone and estrogen. The ovarian cycle is divided into two phases: follicular and luteal.
The follicular phase of the ovarian cycle (days 1-14)
FSH is released from the anterior pituitary
Stimulated by GnRH, FSH is released in a pulsatile fashion from the anterior pituitary. As the level of FSH increases over a 13 to 14-day period, it stimulates the granulosa cells in the cohort of ovarian follicles to synthesize aromatase, which converts androgens produced by the thecal cells to estradiol (one of three different forms of estrogen). FSH also leads to luteinizing hormone (LH) receptor expression on the granulosa cells. Among this group of about 10 antral follicles, a dominant follicle emerges, growing faster than the rest of the cohort and producing higher levels of estrogens.
The ovarian cycle experiences negative feedback
As these follicles develop, they start secreting increasing amounts of inhibin. Inhibin primarily acts to inhibit the secretion of FSH from the anterior pituitary gland. As inhibin levels rise in response to the developing follicles, it provides negative feedback to the pituitary, reducing the production and release of FSH. Estradiol also plays a role in the negative feedback regulation of FSH and as the follicle grows and estradiol increases it causes FSH to decline. The dominant follicle also produces a higher amount of inhibin, a hormone that also participates in the negative feedback regulation of FSH production. Consequently, the other follicles stop maturing and begin degeneration.
The LH surge and ovulation
At this point of the cycle, a striking surge in LH occurs resulting in positive feedback that causes both a slight surge in FSH and an increase in prostaglandins and proteolytic enzymes. The bump in FSH aids in the final maturation of the dominant follicle. At the same time, the prostaglandins and proteolytic enzymes work to release the dominant follicle (also known as follicular rupture), discharging an ovum (illustrated below). This is ovulation which happens approximately 36 hours after the LH surge and is marked by a notable increase in body temperature. It is thought that ovulation from either ovary happens randomly. An unfertilized egg will eventually disintegrate or dissolve in the uterus. It is also important to know that some cycles are anovulatory meaning that ovulation does not occur.
Fertility awareness methods (FAMs) of birth control work to determine the precise time of ovulation to find the relatively fertile and the relatively infertile days in the cycle. The follicular phase length can vary from cycle to cycle therefore monitoring the LH surge and cervical changes is the way to determine when ovulation should occur. The fertility window is referred to as a six-day period with the highest likelihood of conception being approximately five days before ovulation through the day of ovulation. However, Wilcox, Dunson, and Baird (2000) found that conception “during the fertile window is not sufficient to produce pregnancy. Pregnancy depends on the viability of the sperm and egg, the receptivity of the uterus, and other factors that vary widely…”
The luteal phase of the ovarian cycle (days 15-28)
The length of the luteal phase can vary from woman to woman however for each individual it almost always takes the same number of days. After ovulation, the corpus luteum forms within the ovary thanks to remaining FSH and LH transforming the remaining parts of the dominant follicle. The corpus luteum is an endocrine structure that produces primarily progesterone and some estrogen. Progesterone encourages the regrowth of the uterine lining in preparation for the implantation of the fertilized egg. It also blocks the release of FSH and LH to prevent any additional follicles from maturing and rupturing. The level of estrogen produced by the corpus luteum increases to a steady level for the next few days and also participates in a negative feedback loop, blocking the release of gonadotropins. If fertilization does not occur the end of the luteal phase is marked by luteolysis, or the demise of the corpus luteum. This leads to a sharp decline in progesterone.
The menstrual cycle
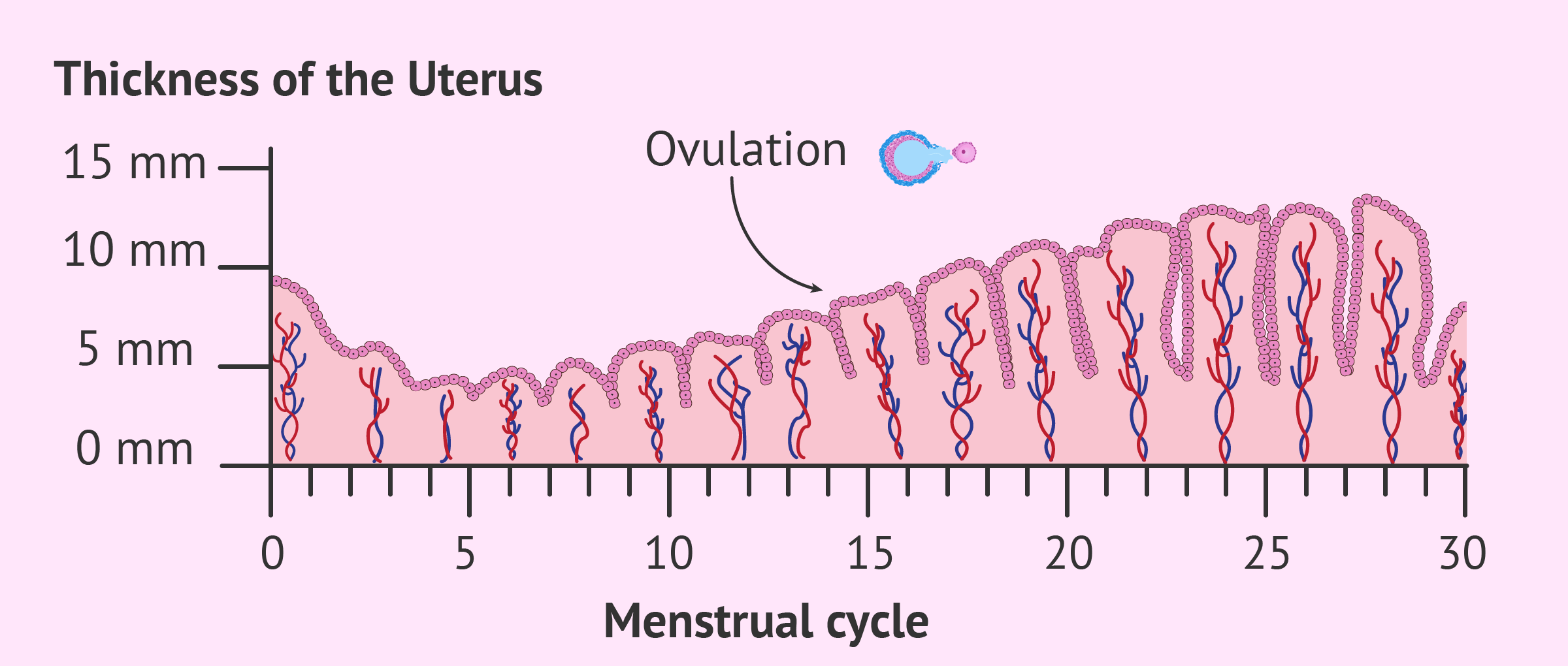
The endometrial lining of the uterus has multiple epithelial layers from superficial (top) to basal (bottom) and it experiences changes throughout the cycle (illustrated below). The changes in the endometrial layers characterize the phases of the menstrual cycle and these endometrial changes are the result of hormonal changes. The menstrual cycle is divided into three phases: menses, proliferative and secretory. The menstrual cycle is typically represented as beginning on the first day of the menses and ending on the last day before the next menses, with the average length being 28 days (coinciding with the ovarian cycle).
The menses phase (days 1-5) of the menstrual cycle
In the absence of pregnancy, a woman experiences menstrual bleeding or what is referred to as “getting her period”. This is the menses phase of the cycle which signifies the beginning of the menstrual cycle. The blood is from the production of menstrual fluid composed of endometrial tissue, red blood cells, and proteolytic enzymes and is emitted through the cervix out of the vagina over an average of five days. Plasmin released from the endometrial cells prevents clotting. The menstrual fluid is the result of the proteolysis of the endometrial lining and cell death due to the plummeting levels of progesterone (thanks to luteolysis). The significant decrease in progesterone also signals to the hypothalamus GnRH neurons to signal to the anterior pituitary, releasing FSH and LH to start the ovarian cycle again. Released by the endometrium, prostaglandins play an integral role during menses by causing vasodilation which narrows the blood vessels of the endometrium, depriving it of oxygen and promoting shedding and stimulating of the superficial layers and causing contractions in the myometrium (muscular layer of the uterus) to help with the shedding of the endometrial lining. Prostaglandins are also responsible for the side effects of cramping and pain associated with menses. As the endometrial shedding occurs, the ovarian cycle begins and within two days regeneration of the surface endometrial epithelium begins, stimulated by estrogen released from the growing follicles.
The proliferative phase (days 4-14) of the menstrual cycle
To prepare the endometrial lining of the uterus for the potential implantation of a fertilized egg, estradiol produced from developing follicles influences the endometrial changes that occur before ovulation. A complete regeneration and restructuring of the endometrial lining with multiple layers and spiral arteries can provide adequate blood flow to the thickened endometrium. This enhanced blood flow provides the necessary oxygen and nutrients that would support possible embryo implantation. Estradiol also triggers the development of progesterone receptors in endometrial cells. The cervix also responds to estrogen by producing a mucoid discharge that lowers the acidity of the vagina and makes the environment more hospitable to potential sperm.
The secretory phase (days 15-28) of the menstrual cycle
Further preparation of the endometrium continues thanks to the influence of progesterone produced by the corpus luteum in the ovary. Progesterone binds to progesterone receptors on the endometrial cells increasing its vascular supply and stimulation of mucosal secretions. It also increases the surface area within the spiral arteries. Progesterone also decreases and thickens the cervical mucus to inhibit sperm motility. If pregnancy occurs, a fertilized ovum is implanted within the endometrium, and the corpus luteum will persist and maintain the hormone levels. However, if no fertilized ovum is implanted, then the corpus luteum degenerates causing a sharp decline in progesterone and estrogen levels. An overview of the general hormone levels and changes in the ovary and uterus over a 28-day cycle is illustrated below.
Problems associated with the cycles
Primary Amenorrhea
If menarche is not reached by age 16 in a young woman who exhibits breast and pubic hair development, she is diagnosed with primary amenorrhea. Klein and Poth (2013) provide a review of the causes of amenorrhea that are attributed to anything that disrupts the onset of the ovarian cycle or prevents the outflow of the shedded endometrial tissue.
Causes of primary amenorrhea
- Outflow tract abnormalities
- Primary ovarian insufficiency
- Hypothalamic or pituitary disorders
- Other endocrine gland disorders
- Chronic disease
- Physiologic (pregnancy) or induced (hormonal contraception)
Secondary Amenorrhea
An absence of menses for greater than three months in females who have had a regular menstrual cycle previously, or an absence of menses for greater than six months in those who have previously had an irregular menstrual cycle is secondary amenorrhea. Klien et al., (2013) note that secondary amenorrhea can often be attributed to polycystic ovary syndrome, problems with the hypothalamus, or premature ovarian failure. Secondary amenorrhea is also associated with low body fat and is common among athletes involved in weight-class and endurance sports (Dadgostar et al. 2009).
Polycystic ovary syndrome
Polycystic ovary syndrome is the formation of multiple cysts within the ovaries due to an imbalance of androgens associated with amenorrhea. Ehrmann (2005) provides a review of this disorder stating that the ovarian theca cells tend to be better at producing testosterone than are normal theca cells in affected women. Furthermore, those with polycystic ovary syndrome also have higher luteinizing hormone pulse frequency which is also associated with greater testosterone production. This is a common disorder affecting up to 10% of all women and evidence indicates a strong genetic component exists.
Abnormal uterine bleeding associated with ovulatory dysfunction (AUB-O)
Anovulation (a lack of ovulation) can be frequent during the first years of the cycle when the HPG axis is immature. Follicular development may start but not complete, nevertheless, estrogens will form and stimulate the uterine lining. Without ovulation, the corpus luteum does not form and progesterone is not released, which serves to stabilize the endometrium until implantation occurs. A lack of progesterone keeps the endometrium in the proliferative phase. This leads to heavy and irregular bleeding known as estrogen breakthrough bleeding. It is also referred to as abnormal uterine bleeding associated with ovulatory dysfunction (AUB-O). The most frequent symptoms are heavy menstrual bleeding, irregular menses, and bleeding between menses (Jones and Sung 2022).
Pre-menstrual syndrome (PMS)
PMS is a combination of physical, emotional, and psychological symptoms that occur in the days or weeks leading up to menstruation. Symptoms can vary widely from mild to severe and may include bloating, breast tenderness, fatigue, irritability, headaches, and mood swings.
Dysmenorrhea
Painful menses are known as dysmenorrhea. Cramping, discomfort, nausea, vomiting, and headaches often accompany menstruation. This menstrual pain can significantly reduce one’s quality of life and productivity. Reviewed by Ju, Jones, and Mishra (2014) dysmenorrhea is quite common with studies reporting over 90% of women surveyed experiencing pain during menses. As for the causes of dysmenorrhea, there have been conflicting results across numerous studies. However, a meta-analysis found a positive relationship exists between emotional stress and painful periods as well as family history and there is an inverse relationship between age, oral contraception use, and number of live births.
Menorrhagia
Menorrhagia is abnormally heavy or prolonged bleeding during menses with over 30% of women reporting this problem. It can limit activities and oftentimes leads to anemia (Van Eijkeren et al. 1989). The cause is associated with fibroids (benign endometrial tumors) and prostaglandin disorders (Duckitt and Collins 2012).
Oligomenorrhea
Cycles that last longer than 35 days or those with less than 9 cycles per year are known as oligomenorrhea. Similar to secondary amenorrhea this is often associated with low body fat and therefore experienced commonly by athletes. Oligomenorrhea is not a problem however by itself yet it can be associated with infertility and endometrium proliferation which may result in abnormal endometrial cells (hyperplasia). Endometrial hyperplasia (abnormal endometrial cells associated with abnormal thickening) may lead to endometrial cancer.
Fibroid Tumors
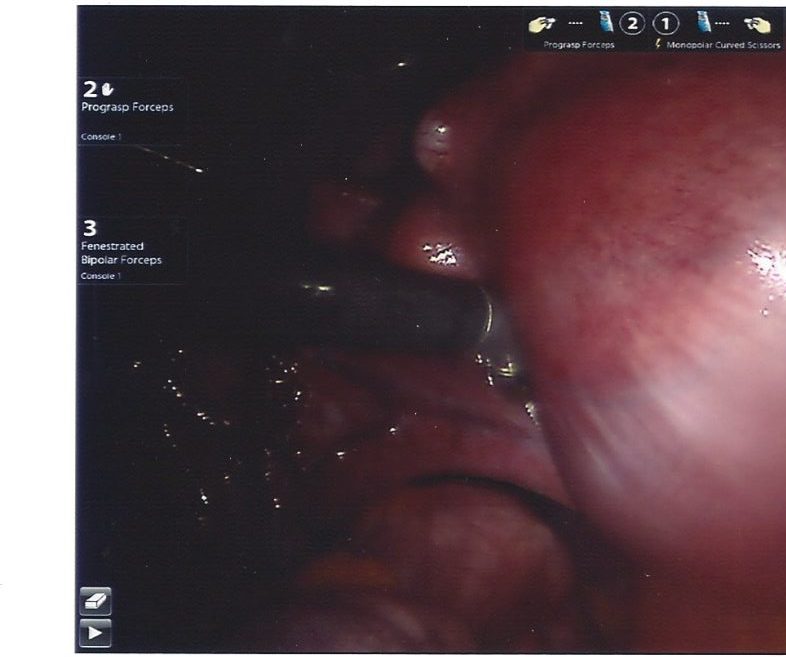
Fibroid tumors, also known as uterine fibroids or leiomyomas, are noncancerous growths that develop in the muscular wall of the uterus. These growths are composed of smooth muscle tissue and fibrous connective tissue and can vary from small, pea-sized nodules to large, grapefruit-sized masses. Fibroids are quite common and “among 15–54 year-old-women, fibroids account for 29% of gynecologic hospitalizations” …with “the lifetime risk for fibroids (intended as the cumulative risk of developing a fibroid by age 50) to be nearly 70% in white women and >80% for black women” (Pavone et al. 2018). The image on right is of a fibroid being removed laparoscopically.
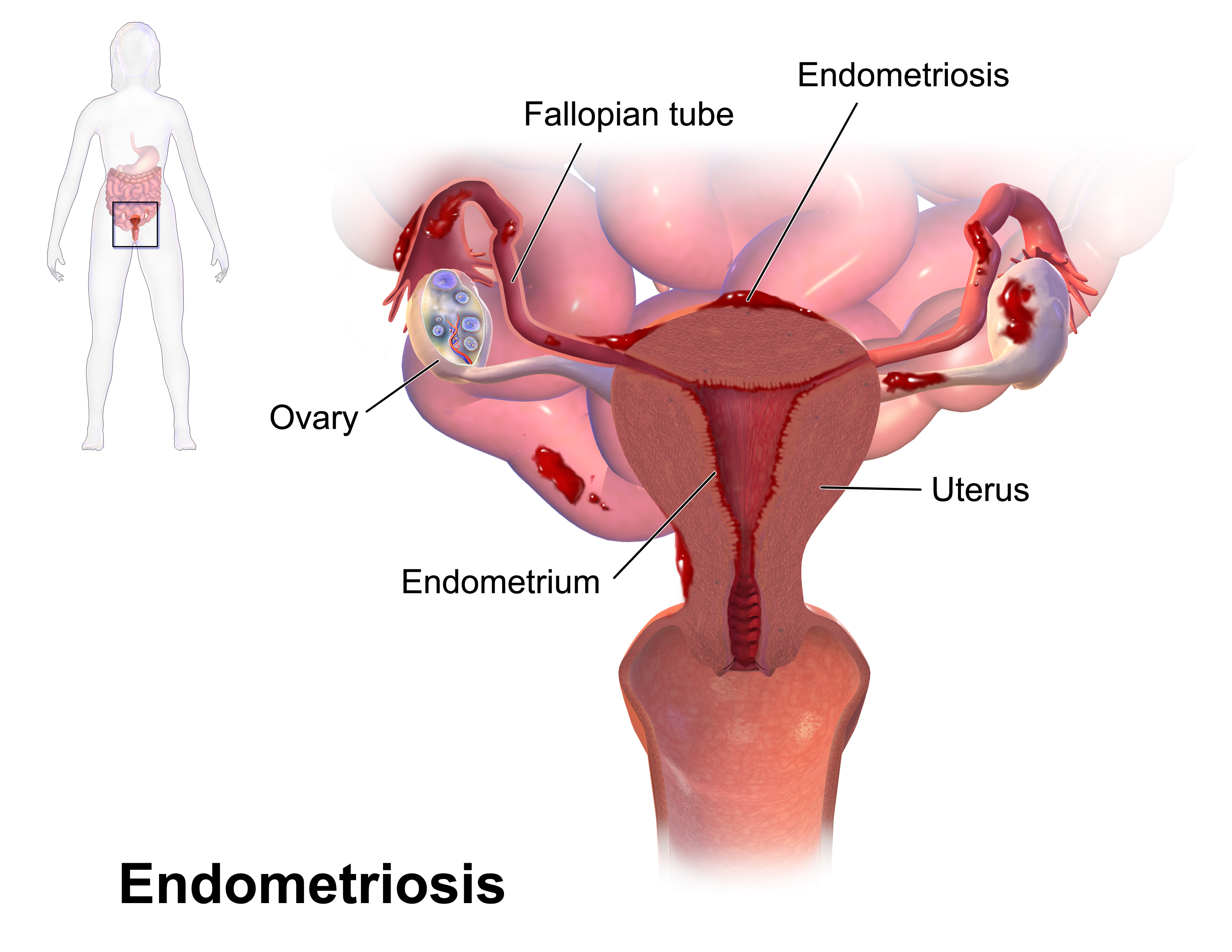
Endometriosis
Endometriosis is characterized by the growth of endometrial tissue outside of the uterus. Endometrial lesions likely go through the fallopian tubes and implant on the peritoneal surface or pelvic organs where they become stimulated by estrogen (Vercellini et al. 2014). This results in chronic inflammation every cycle. Chronic pain is a common symptom associated with the overproduction of prostaglandins and cytokines (Howard 2009).
Society and Menstruation
In this modern era, one would expect menstruation to be viewed openly and positively. However, this is not the case for many, and women are still hampered by taboo. Although improvements have been made, as noted by Ramaiyer et al., (2023) we still have a long way to go “in terms of education, research, and cultural acceptance of menstruation.” The World Health Organization (WHO) has asked for the following actions (1) for menstrual health to be framed with the lens of health and human rights as opposed to solely a matter of hygiene; (2) for menstruating individuals to have access to menstruation information, education, and products; and (3) for menstrual health and related support activities be included in relevant government plans and budgets with measured performance (WHO, 2022). A recent article by Mukherjee in the New York Times highlights the many hurdles menstruating women face, ranging from embarrassment to outright exclusion. For too many there is a lack of access to feminine products. As you can imagine, not having pads or tampons directly impacts a woman’s quality of life. Even in the United States “period poverty” (a lack of access to period products, education, and awareness of menstrual health) is reportedly responsible for 20% of young girls’ absence from school, negatively impacting their education and opportunities (Duvuvier et al., 2022). Changes need to be made both locally and globally because all “women have the right to use safe menstrual products during their monthly menses” (Jaafar et al., 2023).
Estrus
Most mammals do not experience menstruation and instead forgo tissue breakdown and bleeding. Furthermore, ovulation is preceded by conspicuous physiological and behavioral changes to signal a female’s receptivity to mating. Instead, they go through estrus. In some species, ovulation occurs spontaneously in response to hormones, and in others, ovulation is induced by mating called “reflex ovulation”.
The term estrus is derived from the Latin oestrus which means frenzy – referring to the state of a suddenly receptive female experiencing the condition, also known as being in heat. Like menstruation, estrus is also under the regulation of the ovarian cycle and environmental stimuli. For some species, estrus results in outward physiological changes that enable attractiveness and receptiveness to males such as the production of pheromones, genital swelling, and/or reddening. Behavioral changes also may accompany estrus such as vocalization, scent markings, and heightened activity such as the circling of a male observed in wild rabbits (Meyers and Poole 1961). Many species in estrus (e.g. rodents) display a lordosis posture induced by male behavior and increase female receptivity. This behavior is characterized by an elevation of the hind quarters and dorsiflexion of the vertebral column to facilitate penile insertion and aid in fertilization (for review see (Tsukahara, Kanaya, and Yamanouchi 2014).
The four phases of estrus
There are four phases of estrus: proestrus, estrus, metestrus, and diestrus. During proestrus, the ovarian follicles start to grow and estrogen begins to rise influencing the endometrial lining. Some animals may experience vaginal secretions. Next during estrus, the dominant follicle emerges and subsequently ruptures releasing the mature ovum. Metestrus is brief and marked by the formation of the corpus luteum after the follicle ruptures. Progesterone levels increase further preparing the uterine lining for implantation. Progesterone also plays a role in the feedback inhibition of estradiol production, resulting in a decline in estrogen. Diestrus is characterized by a continued rise in progesterone by the corpus luteum. In the absence of pregnancy, the corpus luteum regresses, and diestrus ends. The endometrial lining is not shed but rather reorganized for the next cycle. When the reproductive cycle rests the animal is in anestrus.
Typically a seasonal event, estrus is controlled by sunlight exposure and the release of melatonin which regulates the hypothalamic pulse activity of GnRH. Species that experience estrus once a year are monestrus. Seasonally breeding animals active during the fall are known as “short-day breeders” such as deer and elk, those active during the spring and summer are referred to as “long-day breeders” such as horses and hamsters. Some species lack a defined breeding season and experience estrus throughout the year, which is termed continuous polyestrus (e.g. cows). Moreover, the length of the reproductive cycle varies among species and even within species there is variation.
Why do humans and apes undergo menstruation?
As noted above the vast majority of placental mammals do not undergo menstruation therefore the question remains, why?
Currently, there are three main hypotheses regarding the evolution of menstruation which hinge upon the fact that the process known as decidualization occurs. Ng et al. (2020) define decidualization as ” the functional and morphological changes that occur within the endometrium to form the decidual lining into which the blastocyst implants.” Decidualization is essential for proper priming of the endometrium for a successful pregnancy. In preparation for embryo implantation, the endometrial stromal cells undergo significant changes, including enlargement and transformation into decidual cells. These decidual cells are rich in glycogen and lipids and serve as an energy source for the developing embryo allowing for the successful implantation of an embryo.
The hypotheses on menstruation in the great apes
- Humans and apes have a higher level of embryo invasion compared to other mammals. Therefore menstruation serves as a type of protection against excessive invasiveness and is a “nonadaptive result of endometrial spontaneous shedding” (Emera, Romero, and Wagner 2011)
- Menstruation permits embryo selection by the mother (Clarke 1994)
- Menstruation is a side-effect of the fetal–maternal processes that arrest blood supply to the uterine surface upon pregnancy. Progesterone withdrawal causes the constriction of the uterine spiral arterioles, arresting maternal blood flow to the uterine surface, and the cessation of blood supply to the placenta. This is a vital prerequisite for the delivery of the placenta (Thomas 2019)
There are issues with each of the hypotheses but we need to examine the evolutionary underpinnings of menstruation. As Thomas (2019) points out: half of the World’s human population menstruates, and the total time experiencing menstruation is at least a quarter of each woman’s reproductive years. Menstruation has long been a taboo subject for different religions and cultures, based on ignorance, prejudice, and false information. Yet menstruation is associated with discomfort and health issues for many women. More extensive research to better understand how to manage and alleviate menstrual-related symptoms and conditions is gravely needed.
Think, Pair, Share
There are many gaps in our understanding of the ovarian and menstrual cycles. What are the possible reasons for this?
Every cycle a follicle “wins” and becomes the dominant one that ruptures. Briefly explain this process and develop reasons as to why this process has been selected.
Briefly describe estrogen production during the follicular phase.
How is both positive and negative feedback a part of the cycle regulation?
Describe how the luteal phase of the ovarian cycle and the secretory phase of the menstrual cycle work together to prepare for implantation.
What stimulates estrus and how is this tied into species that have breeding seasons in the spring vs. in the fall?
Deeper Questions
Menstruation evolved independently in shrews and primates. What reasons might there be for selection to result in menstruation over estus and vice versa?
In the literature problems with the menstrual cycle such as premenstrual syndrome and oligomenorrhea are referred to as things women “complain” about. Why might the medical community use the verb “complain” rather than “report” and how does this word impact a physician’s point of view regarding these issues?
If you were designing an oral contraceptive what parts of the ovarian/menstrual cycles would you try to control with it that would be safe and effective?
Why is period poverty still prevalent and how could this be fixed quickly?
Key terms
Amenorrhea
Anestrus
Antral follicle
Atresia
Corpus luteum
Decidualization
Diestrus
Dysmenorrhea
Endometrium
Estradiol
Estrogen
Estrus
Follicle-stimulating hormone
Gonadotropin-releasing hormone
Graafian follicle
Hypothalamus
Inhibin
Lordosis
Luteinizing hormone
Luteolysis
Menarche
Menorrhagia
Monestrus
Oligomenorrhea
Oocyte
Ovulation
Pituitary gland
Polycystic ovarian syndrome
Progesterone
Prostaglandins
Reflex ovulation
References
Clarke, J. 1994. “The Adaptive Significance of Menstruation: The Meaning of Menstruation in the Elimination of Abnormal Embryos.” Human Reproduction 9 (7): 1204–7. https://doi.org/10.1093/oxfordjournals.humrep.a138678.
Critchley, H. O., E. Babayev, S. E. Bulun, S. Clark, I. Garcia-Grau, A. Gregersen, A. Kilcoyne, et al. 2020. “Menstruation: Science and Society.” American Journal of Obstetrics and Gynecology 223 (5): 624–64. https://doi.org/10.1016/j.ajog.2020.06.004.
Dadgostar, H., M. Razi, A.Aleyasin, T. Alenabi, and S. Dahaghin. 2009. “The Relation between Athletic Sports and Prevalence of Amenorrhea and Oligomenorrhea in Iranian Female Athletes.” Sports Medicine, Arthroscopy, Rehabilitation, Therapy & Technology : SMARTT 1 (1): 16. https://doi.org/10.1186/1758-2555-1-16.
DiVall, S. A., and S. Radovick. 2008. “Pubertal Development and Menarche.” Annals of the New York Academy of Sciences 1135.1: 19–28.
Duckitt, K., and S. Collins. 2012. “Menorrhagia.” BMJ Clinical Evidence 2012 (January): 0805.
Duncan, F. E., K. Schindler, R. M. Schultz, C. S. Blengini, P. Stein, S. A. Stricker, G. M. Wessel, and C. J. Williams. 2020. “Unscrambling the Oocyte and the Egg: Clarifying Terminology of the Female Gamete in Mammals.” Molecular Human Reproduction 26 (11): 797–800. https://doi.org/10.1093/molehr/gaaa066.
Duvivier, Cephora. 2022. “Period Poverty: How Access To Feminine Hygiene Products Affects The Psychosocial Development Of Young Women?.” PhD dissertation.
Ehrmann, D. A. 2005. “Polycystic Ovary Syndrome.” New England Journal of Medicine 352 (12): 1223–36. https://doi.org/10.1056/NEJMra041536.
Emera, D., R. Romero, and G. Wagner. 2011. “The Evolution of Menstruation: A New Model for Genetic Assimilation.” BioEssays 34 (1): 26–35. https://doi.org/10.1002/bies.201100099.
Hermes, G. L. 2022. “Martha K. McClintock.” In Biographical History of Behavioral Neuroendocrinology, edited by Randy J. Nelson and Zachary M. Weil, 359–83. Cham: Springer International Publishing. https://doi.org/10.1007/978-3-031-12970-4_36.
Howard, F. M. 2009. “Endometriosis and Mechanisms of Pelvic Pain.” Journal of Minimally Invasive Gynecology 16 (5): 540–50. https://doi.org/10.1016/j.jmig.2009.06.017.
Hsueh, A. J. W., K. Kawamura, Y. Cheng, and B. C. J. M. Fauser. 2015. “Intraovarian Control of Early Folliculogenesis.” Endocrine Reviews 36 (1): 1–24. https://doi.org/10.1210/er.2014-1020.
Jaafar H, Ismail SY, Azzeri A. 2023. “Period Poverty: A Neglected Public Health Issue”. Korean J Fam Med. 44(4):183-188. doi: 10.4082/kjfm.22.0206. Epub 2023 May 16. PMID: 37189262; PMCID: PMC10372806.
Jones, K., and S. Sung. 2022. “Anovulatory Bleeding.” https://www.ncbi.nlm.nih.gov/books/NBK549773/.
Ju, H., M. Jones, and G. Mishra. 2014. “The Prevalence and Risk Factors of Dysmenorrhea.” Epidemiologic Reviews 36 (1): 104–13. https://doi.org/10.1093/epirev/mxt009.
Kaplowitz, P. B. 2008. “Link between Body Fat and the Timing of Puberty.” Pediatrics 121 Suppl 3 (February): S208-217. https://doi.org/10.1542/peds.2007-1813F.
Klein, D. A., and Merrily A. Poth. 2013. “Amenorrhea: An Approach to Diagnosis and Management.” American Family Physician 87 (11): 781–88.
McGee, E. A., and A. J. W. Hsueh. 2000. “Initial and Cyclic Recruitment of Ovarian Follicles*.” Endocrine Reviews 21 (2): 200–214. https://doi.org/10.1210/edrv.21.2.0394.
Meyers, K., and W. E. Poole. 1961. “A Study of the Biology of the Wild Rabbit, Oryctolagus Cuniculus (L) in Confined Populations. II. The Effect of Season and Population Increase on Behavior” CSIRO Wildlife Research 6: 1–41.
Ng, S., G. A. Norwitz, M. Pavlicev, Tamara Tilburgs, Carlos Simón, and Errol R. Norwitz. 2020. “Endometrial Decidualization: The Primary Driver of Pregnancy Health.” International Journal of Molecular Sciences 21 (11). https://doi.org/10.3390/ijms21114092.
Oktem, O., and B. Urman. 2010. “Understanding Follicle Growth in Vivo.” Human Reproduction 25 (12): 2944–54. https://doi.org/10.1093/humrep/deq275.
Pavone, D., S. Clemenza, F. Sorbi, M. Fambrini, and F. Petraglia. 2018. “Epidemiology and Risk Factors of Uterine Fibroids.” Alternatives to Hysterectomy 46 (January): 3–11. https://doi.org/10.1016/j.bpobgyn.2017.09.004.
Ramaiyer, M., Lulseged, B., Michel, R. et al. 2023. “Menstruation in the USA.” Curr Epidemiol Rep (10):186–195. https://doi.org/10.1007/s40471-023-00333-z
Rees, M. 1997. “The Abnormal Menstrual Cycle.” In Women’s Health, 4th ed., 308.
Richards, J.S. 2018. “Chapter One – The Ovarian Cycle.” In Vitamins and Hormones, edited by Gerald Litwack, 107:1–25. Academic Press. https://doi.org/10.1016/bs.vh.2018.01.009.
Shalev, D., and P. Melamed. 2020. “The Role of the Hypothalamus and Pituitary Epigenomes in Central Activation of the Reproductive Axis at Puberty.” Molecular and Cellular Endocrinology 518 (December): 111031. https://doi.org/10.1016/j.mce.2020.111031.
Stern, K., and M. K. McClintock. 1998. “Regulation of Ovulation by Human Pheromones.” Nature 392 (6672): 177–79. https://doi.org/10.1038/32408.
Tanner, J. M., and R. H. Whitehouse. 1976. “Clinical Longitudinal Standards for Height, Weight, Height Velocity, Weight Velocity, and Stages of Puberty” Archives of disease in childhood51: 170–79.
Thomas, V. 2019. “The Link Between Human Menstruation and Placental Delivery: A Novel Evolutionary Interpretation” Bioessays 41 (6). https://doi.org/10.1002/bies.201800232.
Tsukahara, S. ,M. Kanaya, and K. Yamanouchi. 2014. “Neuroanatomy and Sex Differences of the Lordosis-Inhibiting System in the Lateral Septum.” Frontiers in Neuroscience 8: 299. https://doi.org/10.3389/fnins.2014.00299.
Uenoyama, Y., N. Inoue, S. Nakamura, and H. Tsukahara. 2019. “Central Mechanism Controlling Pubertal Onset in Mammals: A Triggering Role of Kisspeptin.” Frontiers in Endocrinology 10: 443523. https://doi.org/10.3389/fendo.2019.00312.
Van Eijkeren, M. A., G. C. M. L. Christians, J. J. Sixma, and A. A. Haspels. 1989. “Menorrhagia: A Review.” Obstetrical & Gynecological Survey 44 (6). https://journals.lww.com/obgynsurvey/Fulltext/1989/06000/Menorrhagia__A_Review.3.aspx.
Vercellini, P., P. Viganò, E. Somigliana, and L. Fedele. 2014. “Endometriosis: Pathogenesis and Treatment.” Nature Reviews. Endocrinology 10 (5): 261–75. https://doi.org/10.1038/nrendo.2013.255.
Walvoord, E. C. 2010. “The Timing of Puberty: Is It Changing? Does It Matter?” Journal of Adolescent Health 47 (5): 433–39. https://doi.org/10.1016/j.jadohealth.2010.05.018.
Wilcox, A. J., D. Dunson, and D. D. Baird. 2000. “The Timing of the ‘Fertile Window’ in the Menstrual Cycle: Day Specific Estimates from a Prospective Study.” BMJ (Clinical Research Ed.) 321 (7271): 1259–62. https://doi.org/10.1136/bmj.321.7271.1259.
Suggested Readings
“In the Flo: Unlock Your Hormonal Advantage and Revolutionize Your Life” by Alisa Vitti – This book explores how understanding and harnessing your hormonal cycle can lead to better health, well-being, and productivity.
“Period Power: Harness Your Hormones and Get Your Cycle Working For You” by Masie Hill – This is a practical blueprint for aligning daily life with the menstrual cycle, to give women a no-nonsense explanation of what the hell happens to their hormones every month and how they can use each phase to its full advantage.
“Cycle Savvy: The Smart Teen’s Guide to the Mysteries of Her Body” by Toni Weschler – Designed for teenagers and young adults, this book offers an accessible and informative overview of the menstrual cycle and reproductive health.
“The Menstrual Cycle: Physiology, Reproductive Disorders, and Infertility” by Irina Szmelskyj and Jackie Bernardi – This book provides a detailed exploration of the menstrual cycle from a medical perspective, covering topics related to reproductive disorders and infertility.
“Cycles: The Science of Periods, Why They Matter, and How to Nourish Each Phase” by Amy J. Hammer – Registered nurse Amy Hammer arms you with a strong foundation in physiology and hormonal health, explores historical and socio-cultural aspects of women’s health, and reimagines the phases of the menstrual cycle as aligning with the four seasons to provide a detailed guide for living well in your body.
Interesting Podcasts
NPR’s Life Kit This is the period talk you should have gotten
PsycheCentral: The menstrual cycle, stigma and mental health
