24
Madeline DeFlippo and Megan Parham
Introduction
Keywords
genome
gene
germline
gene-editing
CRISPR-Cas9
nuclease
biomedicine
HIV
embryo
mutation
DNA
RNA
cancer
cell
Learning Objectives
- Understand the basic science behind gene-editing
- Learn the goals and objectives of the Human Genome Project and the revolutionary results
- Understand Dr. He Jiankui’s Germline Editing experiment and the resulting controversy surrounding his results
- Understand what CRISPR-Cas9 is.
- The potential gene editing technology for treating diseases, such as Sickle Cell Disease.
- The limitations of CRISPR and gene editing technology.
The inspiration fueling the search for the human genome dates back to as early as 1900 when the principles of Gregor Mendel’s laws of inheritance were applied to human generations instead of those of garden peas (Kevles). After discovering the location of human genes within chromosomes, the scientific search for the human genome was popularly fueled by the social attraction of improving the quality of the human race. While this “cluster of ideas and activities” was first constructed by Plato, it is modernly known as “eugenics” thanks to Francis Galton. While many scientists were invested in drafting the human genome and germline editing for medicinal purposes, ethical and moral implications birthed from the possibility of modern eugenics made easier through technological advancements (Kevles). This prospect is heavily related to that of the commonly dubbed scientific breakthrough, “designer babies.” CRISPR technologies have recently given rise to the possibility of editing human genes within embryos, thus eliminating hazardous or unwanted genes. Thus, the concept of genome germline editing in human embryos, while a major scientific discovery, has also sparked international controversy. Many people worldwide are concerned that this gene-editing technology could be used as a tool for eugenic interests. In contrast, others debate on the ethics of utilizing CRISPR technologies for primarily medicinal purposes. In late 2018, many international alarms were flagged by Dr. He Jiankui’s Human Genome experiment in which he used CRISPR technology to genetically immunize twin girls against HIV. To date, the girls are healthy and remain immunized. However, although his experiment was successful, Dr. He Jiankui ignited an international debate on his experiment’s ethics. Modern developments in gene editing technologies have fueled several international controversies regarding the completion of the human genome draft and the prospect of designing DNA sequences of human offspring (Kevles).
Introduction to Gene Editing
Key Takeaways
Gene editing is defined as the manipulation of organic genetic material by replacing, deleting, or inserting DNA sequences. This technology depends solely on programmable nucleases.
Nucleases are customized proteins capable of acting on specific segments of DNA. Current Gene editing technologies are possible due to the development of programmable nucleases. The first programmable nuclease used for human gene editing, known as the meganuclease, was first used in 1944.
As of 2013, the most promising programmable nuclease is clustered regularly interspaced short palindromic repeats technology, popularly known as CRISPR.
Recent studies show that DNA double-strand breaks are expected to be the central method for gene editing within the next ten years due to the rise in CRISPR technologies. The development of CRISPR technologies inspires hope for future treatments of human diseases, such as cancer, infectious viruses, cardiovascular diseases, muscular dystrophy, and many more.
The Human Genome Project
Key Takeaways
The human genome comprises all the various genes in the cells of human beings. In 1990, the Human Genome programs of the National Institutes of Health and the department of energy collaborated to design a research plan for drafting the human genome. This plan, mapped for five years, was an international effort to improve human DNA models and acquire genetic information. During the project’s completion, there were several significant advances in genetic technologies, such as mapping, sequencing, and gene identification. Other revolutionary developments include the development of new genetic markers and improved cloning DNA fragments. Many countries and corporations contributed to the Human Genome Project, such as the United Kingdom, Australia, and Japan. International coordination was highly encouraged in the field of gene editing, which still heavily depends on international cooperation and sharing. The Human Genome Project was first launched in the United States in 1990, and the draft was completed in 2003. The project plan outlined five years of various goals, such as developing efficient methods for identifying genes, placing known genes on physical maps, developing rapid genotyping technologies, and identifying more efficient genetic markers.
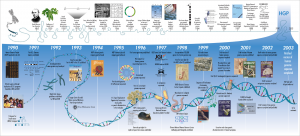
“File: Human Genome Project Timeline” by Darryl Leja, NHGRI is licensed under CC BY 2.0
This is a visual representation of the timeline of the Human Genome Project. As you can see, the main timeline starts in 1990 when the project was first launched in the United States and ending in 2003 with the completion of the first human genome draft. The Human Genome Project resulted in the basic human DNA sequence that has inspired a multitude of secondary studies that have improved the understanding of the human genome. To date, the genome project has revolutionized biomedical research by allowing for the isolation of genes linked to several diseases, such as Huntington’s Disease, myotonic dystrophy, and fragile X syndrome. The human genome project has also resulted in an improved understanding of genes associated with predispositions to several cancers and neuroinflammatory diseases.

“Francis Collins, M.D., Ph.D., announces the successful completion of the Human Genome Project” by Ernie Branson, National Human Genome Research Institute is licensed under CC BY 2.0
Above, Francis Collins, leader of the Human Genome Project, announced the successful completion of the Human Genome Project in 2003. Even a decade after the release of the human genome’s draft sequence, studies are being conducted further to understand the human genome’s architecture and function. Although major developments have been made, gene-editing technology is still relatively new and continues to be internationally researched. In response to the Human Genome Project, another international effort, the International HapMap Project, has been constructed in order to gain more comprehension on genetic variation–development in this field is imperative to genome associated studies involving complex diseases. However, along with great technological advancement comes great controversy. Recently, China has allowed for scientific innovations to travel from the laboratory to the living room. Civilians are allowed to access technological innovations, such as gene-editing technology. While this approach fuels discoveries in science and medicine, it has also sparked challenges within ethics.
CRISPR-Cas9 Technology
Key Takeaways
CRISPR stands for cluster regularly interspaced short palindromic repeats (Cai et al. 2016). It is a method of gene editing that is used to medically edit mutations in the genome. This editing allows the genes that are being expressed to be changed, whether this is through suppression or deletion of the gene. This process was modeled after a natural genome editing system that occurs in bacteria (MedlinePlus, n.d.). The use of CRISPR in the lab happens very similarly to the natural bacteria process.
The diagram below, created by J. Levin illustrates the process of CRISPR-Cas9. First, the guide RNA is created which is seen in grey in the diagram. The guide RNA has a specific sequence that will lead it to bind to a specified target sequence of DNA in the genome. The RNA will also bind to the Cas9 enzyme which will cut the DNA at the specific location after it has been recognized.. After the DNA is cut, the cell’s own DNA repair mechanisms are used to either silence the gene that is the target or the gene that is a problem can be replaced with a correct segment of DNA or a new gene can be introduced in place of the one that was a problem. The replacement gene is seen in yellow. The pink section of the DNA is the PAM sequence, which allows the Cas9 to actually bind to the target DNA. What is seen at the bottom of the diagram is potential applications of this technological development. It could be used in food and livestock modification; gene drive, which is editing genes that will affect evolution; gene therapy for treating diseases; human germ line which would change the genes of future generations; and designer organisms, or choosing what characteristics we would like organisms to have. The gene therapy option will be discussed in more detail in this chapter.
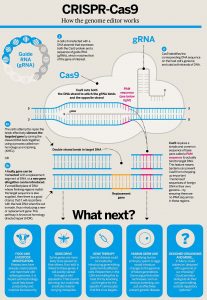
“CRISPR-Cas9 Biologist” by J Levin W is licensed under CC BY-SA 4.0
Disease Treatment
Key Takeaways
Overview of Potential
Each of the following are genetic diseases that have the potential to be treated with gene therapy: Chronic granulomatous disease, Duchenne Muscular Dystrophy, Hemophilia, ß-Thalassemia, Cystic Fibrosis, Barth Syndrome, Sickle Cell Disease, and Huntington’s Disease (Cai et al. 2016). Sickle Cell Disease has been tried in humans (Stein, June 2020). Huntington’s disease has been studied in mice (Yang et al. 2017). As of March of 2020, clinical trials had begun for CRISPR treatment of Sickle Cell Disease, two for β-thalassemias, and Leber congenital.
Sickle Cell Disease
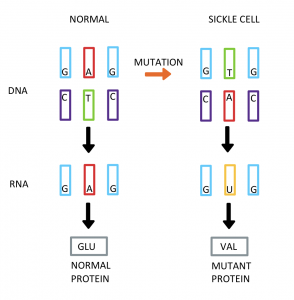
Sickle Cell Disease is a genetic disease that is caused by a single mutation (CDC, n.d.). This mutation can be seen in Diagram 1.2. The DNA mutation occurs when the nitrogenous base adenine is replaced with thymine (Caldwell et al., n.d.). This causes the DNA that translates to RNA to create the incorrect protein. The correct protein would be glutamate. The mutation codes for the amino acid valine (Caldwell et al., n.d.). This mutation in which codes for the wrong amino acid is problematic because a chain of amino acids determines the structure of a protein. When it comes to proteins, structure determines functions (Petsko and Ringe, 2004). Therefore, when the structure of the protein is incorrect due to this change in amino acid, it will not serve the correct function. This is what causes the red blood cells that are characteristic of the disease to develop. The sickled blood cells can be seen in Diagram 1.3. Normally, blood cells are round in shape. Sickle Cell Disease has misshapen red blood cells that causes the cells to die early and clog blood flow which is extremely painful (CDC, n.d.).
Sickle Cell Disease is one that has been tried and shown positive results in humans. Victoria Gray was diagnosed with this disease which was extremely painful for her (Stein 2020). She had been under the impression that she was going to miss many milestones in the lives of her children. In 2019, she received CRISPR treatment for her disease. Scientists removed some of her bone marrow cells and used CRISPR to edit the mutated gene as seen in Diagram 1.2. Their goal was to edit the genes so they would produce a protein called fetal hemoglobin. Restoring the fetal hemoglobin production in Victoria Gray’s body would allow her cells to produce blood cells that were not sickled. They were hoping to have about 20% of fetal hemoglobin restored, but in the summer of 2020 during a check up, it was found that her body had produced about 46% fetal hemoglobin. Additional results showed that about 81% of her cells had the intended genetic change. Victoria Gray had almost all of her symptoms relieved. These results were higher than expected and hoped that these results will continue to show in more trials in the future. The results cannot necessarily be generalized and assumed to present the same in other patients, however they are promising results and it is important to continue to carry out these studies (Stein 2020).
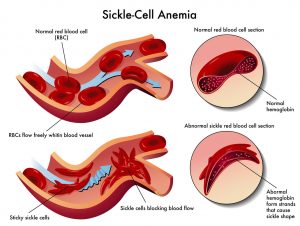
“Risk Factors for Sickle Cell Anemia” by Diana Grib is licensed under CC BY-SA 4.0
Huntington’s Disease
Huntington’s disease is caused by a mutation of the HTT gene on chromosome 4 (Alzheimer’s Association, n.d.). It is characterized by uncontrolled movement of arms, legs, head, face, and upper body. These are caused by brain changes that also account for changes in mood, depression, anxiety, anger, irritability and obsessive compulsive disorder (Alzheimer’s Association, n.d.). This defective HTT gene codes for a protein called huntingtin. Current treatments have the intention of reducing symptoms, but do not get to the root of the issue, which is the genetic mutation. This is where CRISPR is important.
A study of Huntington’s was done in mice. In these mice, it was found that shutting off the expression of the HTT gene relieved some of the neurological symptoms of the disease (Yang et al. 2017). Editing the gene could help manage symptoms in humans, if the edit occurs the way that it was able to happen in mice. Similarly to sickle cell disease, more trials need to be done. However, this is another example of the potential that CRISPR has for treating genetic diseases.
Cancer Treatment
Key Takeaways
Cancerous cells are the result of genes that make them reproduce uncontrollably. This is when tumors develop as they are a build up of these cells. These cells also do not die at the rate they are supposed to (National Cancer Institute, n.d.). Scientists have been looking for ways to fix the mutations in DNA that cause cancer. Although, each treatment must be individualized and changed for each patient as cancer presents differently in each body (Lo et al. 2005). However, CRISPR is much faster and cost effective than research that has been done in the past (Yin et al. 2019). This technology of gene editing could change the developments of cancer treatment because it gets to the root of the problem and is less likely to kill additional cells that could be healthy. The CRISPR treatment would target where the issue of cancer begins (National Cancer Institute, n.d.). CRISPR is also being used in general study of cancer and how the genetic aspect of it works. This approach research could happen faster than previous methods have (Yin et al. 2019).
Limitations of CRISPR
Key Takeaways
Testing and studies of CRISPR are still limited. It has not been very long since serious trials and studies have begun. This technology could also lack precision. It is difficult to use a technology that is this specific, and there is always room for human error. There is potential for genetic disruption to occur beyond what was intended (Uddin et al. 20200. This can be very dangerous and even deadly, such as causing apoptosis, or cell death that is not intended. One example of the potential dangers of CRISPR was present when a patient died four days after getting treated for ornithine transcarbamylase or OTC deficiency (Uddin et al. 2020). This clearly is not what scientists wanted to see with this technology. Although, it is anticipated that it will not work one hundred percent of the time. The lack of firm conclusions does not allow scientists to generalize results and use CRISPR as large scale treatments, however there is a great deal of potential for treating with gene editing (Uddin et al. 2020).
Germline Genome Editing; “Designer Babies”
Key Takeaways

“Chinese biomedical researcher Dr. Jiankui He” by The He Lab, Wikipedia Commons is licensed under CC BY 3.0 / A derivative from the original work
In late 2018, Dr. He Jiankui (figure above), a young Chinese scientist, announced the birth of the world’s first gene-edited babies–twin girls genetically immune to HIV. He Jiankui, a U.S trained biophysicist, edited the heritable germline genome of embryos and implanted them in the human reproductive system. Thus, the fear of “designer babies” was born. Controversy sparked around the globe–many called Dr. He and his team a band of “rogue scientists” that breached the ethical standards of biomedical research. Although his human genome experiment arguably revolutionized the field of biomedicine and gene editing, it also provoked a series of ethical and socio-cultural investigations. The debate regarding the regulation of CRISPR technologies is heavily fueled by the fear resulting from Dr. He’s human genome experiment — CRISPR technologies potentially allow civilians to access germline genome editing technology or the capability of making inheritable changes to human embryos. This technology is already being advertised as assisted reproduction technology, similar to modern fertility services allowing for the selection of embryos bearing specific characteristics. At an international level, the regulation of these technologies depends on culture and style of government–thus raising the possibility of germline genome editing being incorporated into the “reproductive tourism” phenomenon, in which individuals cross borders to access assisted reproductive services that are not provided by their government, currently common in the forms of surrogacy and donor gametes.

“International Summit on Human Gene Editing” by Pam Ridson, The National Academies is licensed under CC BY-NC-SA 2.0
Thus, scientists are faced with ethical and moral concerns regarding this advancing technology–it is imperative that CRISPR technology be used responsibly and appropriately without hindering further research or development. The recent birth of the world’s first genetically edited babies has sparked debate on the commercial deployment of germline genome editing through private clinics, or if the technology should be provided publicly–while the boundaries of germline editing are defined through the Human Gene Summits (fig 4) and similar meetings, the therapeutic and reproductive applications have yet to be discussed. However, many scientists agree that boundaries for germline genome editing technology should be tailored according to the public census.
Chapter Summary
- Gene-editing is the manipulation of organic materials. Current gene editing technologies are possible due to the discovery of programmable nucleases, the most popularly known being CRISPR Cas9.
- The Human Genome Project aimed to increase the human understanding of the genome and resulted in improved treatments for several diseases, including Huntington’s disease and several cancers.
- Germline genome editing in human embryos has sparked an onslaught of biomedical developments, but also fueled international controversy over the commercial deployment of the technology.
Exercises
- What were the main objectives of the Human Genome Project?
a. To create a physical map of the human genome
b. To discover the location of genetically inherited disease-linked genes
c. To gain a better understanding of the human genome
d. All of the above
2. Which disease were the genetically-edited twins of Dr. He Jiankui’s experiment immunized to?
a. Huntington’s disease
b. Pancreatic Cancer
c. HIV
d. Diabetes
3. Who publicly revealed the completion of the Human Genome draft?
a. Albert Einstein
b. Francis Collins
c. Elon Musk
d. Hillary Clinton
4. What is the main argument revolving around germline genome editing?
5. What does CRISPR stand for?
6. CRISPR technology was modeled after a natural gene editing process in what kind of cells?
7. List three diseases that have the potential to be treated with CRISPR.
8. What disease showed promising results when treated with CRISPR in a human patient?
9. List two potential limitations of CRISPR technology
Answers
- D
- C
- B
- Cluster regularly interspaced short palindromic repeats,
- Bacteria,
- Answers may include any three of the following: Chronic granulomatous disease, Duchenne Muscular Dystrophy, Hemophilia, ß-Thalassemia, Cystic Fibrosis, Barth Syndrome, Sickle Cell Disease, and Huntington’s Disease,
- Sickle Cell Disease,
- Answers may include any two of the following: there have not been many trials, human error could occur, patients have died, cause accidental cell death, lack of firm conclusions.
References
Adams, Mark D., et al. “Complementary DNA sequencing: expressed sequence tags and human genome project.” Science 252.5013 (1991): 1651-1656
Alzheimer’s Association. (n.d.). Huntington’s Disease. Retrieved from Alzheimer’s Association: https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/huntington-s-disease?utm_source=google&utm_medium=paidsearch&utm_campaign=google_grants&utm_content=types_of_dementia&gclid=CjwKCAiAnIT9BRAmEiwANaoE1cZLne7URF_IoxNwzcSksYBLNJbT6fU
Cai, L., Fisher, A. L., Huang, H., & Xie, Z. (2016). CRISPR-mediated genome editing and human diseases. Genes & Diseases, 244-251.
Caldwell, R., Lindberg, D., Scotchmoor, J., Thanukos, A., Frankel, J., & Smith, D. (2020). A case study of the effects of mutation: Sickle cell anemia. Retrieved from Understanding Evolution: https://evolution.berkeley.edu/evolibrary/article/0_0_0/mutations_06
Center for Disease Control and Prevention. (2019). Sickle Cell Disease. Retrieved from CDC: https://www.cdc.gov/ncbddd/sicklecell/facts.html
Collins, Francis, and David Galas. “A new five-year plan for the US Human Genome Project.” Science 262.5130 (1993): 43-46
“Human Genome Project.” Columbia Electronic Encyclopedia, 6th Edition, Feb. 2020, p. 1. EBSCOhost, search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=134520806.
Kevles, Daniel J., and Leroy E. Hood, eds. The code of codes: Scientific and social issues in the human genome project. Harvard University Press, 1992Lo, H.-W., Day, C.-P., & Hung, M.-C. (2005). Cancer-specific gene therapy. Advances in Genetics, 233-255.
Morrison, M., de Saille, S. “CRISPR in context: towards a socially responsible debate on embryo editing”. Palgrave communications, 5(110). https://apps-webofknowledge-com.libproxy.clemson.edu/full_record.do?product=UA&search_mode=GeneralSearch&qid=6&SID=5DkdyQleGTbF9bTC37C&page=1&doc=2
Naidoo, N., Pawitan, Y., Soong, R. et al. Human genetics and genomics a decade after the release of the draft sequence of the human genome. Hum Genomics 5, 577 (2011). https://doi-org.libproxy.clemson.edu/10.1186/1479-7364-5-6-577
NCI Staff. (2020, July 27). How CRISPR is changing cancer research and treatment. Retrieved from National Cancer Institute: https://www.cancer.gov/news-events/cancer-currents-blog/2020/crispr-cancer-research-treatment
Nie, Jing-Bao, et al. “Conflict of Interest in Scientific Research in China: A Socio-ethical Analysis of He Jiankui’s Human Genome-editing Experiment.” Journal of Bioethical Inquiry 17.2 (2020): 191-201
Petsko, G. A., & Ringe, D. (2004). Protein structure and function. New Science Press Ltd.
Stein, R. (2020, June 23). A year in, 1st patient to get gene editing for sickle cell disease is thriving.
Segers, Seppe, et al. “0RW1S34RfeSDcfkexd09rT2in Vitro1RW1S34RfeSDcfkexd09rT2 Gametogenesis and the Creation of ‘Designer Babies’: CQ.” Cambridge Quarterly of Healthcare Ethics 28.3 (2019): 499-508. ProQuest. 20 Oct. 2020 .
U.S. National Library of Medicine. (n.d.). What are genome editing and CRISPR-Cas9? Retrieved from MedlinePlus: https://medlineplus.gov/genetics/understanding/genomicresearch/genomeediting/#:~:tex=CRISPR%2DCas9%20was%20adapted%20from,(or%20closely%20related%20ones)
Uddin, F., Rudin, C. M., & Sen, T. (2020). CRISPR gene therapy: applications, limitations, and implications for the future. Frontiers in Oncology, 1387.
Wu, S.-S., Li, Q.-C., Yin, C.-Q., Xue, W., & Song, C.-Q. (2010). Advances in CRISPR/Cas-based gene therapy in human genetic diseases. Theranostics, 4374-4382.
Yang, S., Chang, R., Yang, H., & Zhao, T. (2017). CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J Clin Invest, 2719-2724.
Yin, H., Xue, W., & Anderson, D. G. (2019). CRISPR-Cas: a tool for cancer research and therapeutics. Clinical Oncology, 281-295.
