23
John Carpin and Maggie Segars
Introduction
Keywords
- Stem cells
- Adult Stem Cell
- Induced Pluripotent Stem Cell
- Embryonic Stem Cell
- Blastocyst Stage
- Self Renewal
- Differentiation
- Potency
- Epidermal
- Hematopoietic
- Adipose
- Neural
- Mesenchymal
- Transfected
- Retrovirus
- Tissue engineering
- Organogenesis
- Stem cell theory of cancer
- Rejection
- Cancer
- Vascular graft
- Chemothrapy
Learning Objectives
- Understand the different types of stem cells
- Understand the progression of stem cell technology in medicine
- Understand the different ways they can be implemented into medical procedures
- Understand the resistance to implementing stem cell technology
Over the last 40 or so years, stem cell research and therapy has grown drastically. There are three different types of stem cells that can be utilized for treatments and therapies. This chapter will go into detail on the types of stem cells and the ways in which they can be implemented into medical therapies.
Stem cells are cells that have the potential to develop into any different type of cell in the body. They can be isolated from

“Stem Cell Division” by Genome Research Limited is licensed under CC BY 4.0
humans and used to treat all sorts of conditions or diseases. There are three major categories of stem cells. The first are adult stem cells which are isolated from fully developed organs or tissues. The second type are induced pluripotent stem cells which are a form of isolated adult stem cells and the last are embryonic stem cells which originate from an embryo during its blastocyst stage, which occurs 3-5 days after fertilization. Self renewal is defined as the ability to recreate oneself. Differentiation means to become different over the stages of growth. Stem cells have the ability to self renew and differentiate. They are unspecialized cells that possess the potential to become specialized. They are being researched in hopes to treat conditions such as Alzheimer’s disease, Parkinson’s disease, diabetes, and arthritis (Scadden). A large component of a stem cell is their potency. This refers to the ability of the cell to differentiate into a specialized cell. There are 5 different types of potency. Totipotent cells are cells in zygotes (fertilized cells) that have the potential to differentiate into any type of cell. Pluripotent cells are embryonic stem cells and induced pluripotent stem cells that differentiate into most types of cells that make up the body. Multipotent cells such as hematopoietic stem cells and mesenchymal stem cells differentiate into many cells within a closely related family. Oligopotent cells like lymphoid stem cells and myeloid stem cells differentiate into a few types of cells with the same function. Finally, unipotent cells such as muscle cells only differentiate into one cell type.
Types of Stem Cells
Key Takeaway
Adult stem cells are cells that are undifferentiated found in the brain, blood, intestine, skin, heart and liver. They are able to be undifferentiated because they live in a microenvironment known as a niche that clocks them from becoming differentiated. Adult stem cells have the ability to maintain and repair the tissues where they reside. They are typically multipotent cells which means they can differentiate into multiple different lineages. There are several different adult stem cells.
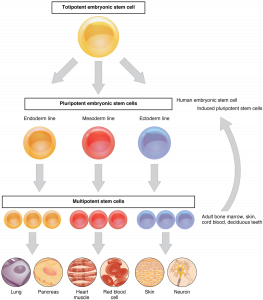
“Feature Stem Cell” by OpenStax College is licensed under CC BY 3.0
There are epidermal stem cells, hematopoietic stem cells, cancer stem cells, adipose stem cells, neural stem cells, and mesenchymal stem cells. Epidermal refers to the outermost layer of the skin, hematopoietic refers to blood cellular component, adipose refers to body tissue used to store fat, neural refers to the nerves from the brain and spinal cord, and mesenchymal refers to the bone marrow. All of these cells can be used in different ways to help treat different diseases and conditions.
Induced pluripotent stem cells are stem cells that are generated directly from adult stem cells. They are produced by isolating the cells from the host and culturing, or growing, them in a stable environment. Genes that allow for the cell to be reprogrammed to its desired function are then transfected, introduced to a genetic material by infecting the cell, through the use of a retrovirus (Yu, 2007). Retroviruses are a group of RNA viruses which insert a DNA copy of their genome into the host cell in order to replicate. The cells are then harvested and cultured again. They are then considered induced pluripotent stem cells and they behave like embryonic stem cells
Embryonic stem cells exhibit practically unlimited developmental potential. The stem cells are derived from embryos when they are at their developmental stage. They are 3-5 days old during the blastocyst stage and are made up of approximately 150 cells. This is when they must be derived. They are pluripotent cells meaning they can differentiate into any cell in the body. They also maintain a self renewal property meaning they can differentiate into even more stem cells.
Applications
Key Takeaway
Adult stem cells have a wide variety of medical applications. Epidermal stem cells are often used in burn therapy, healing of chronic wounds, treatment of genetic disorders, and hair follicle regeneration. Hematopoietic stem cells are used to treat bone disorders, blood disorders and blood cancers. Cancer stem cells are used in therapies by targeting the stem cell in order to inhibit the growth of a tumor. Adipose stem cells are typically used in breast reconstruction as well as lipoatrophy, treatment of cardiovascular disease (Segers, 2008), hair growth therapy, neural therapy, and treatment of different forms of arthritis. Neural stem cells are used to correct spinal cord injuries and are used in treatments of central nervous system disorders (Kim, 2009). Finally, mesenchymal stem cells are used in the treatment of multiple sclerosis, heart disease, tendon healing, bone healing, and neuron healing (Wei, 2013).
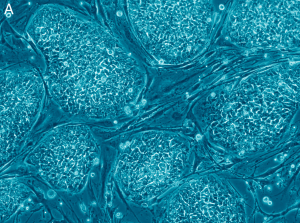
“Feature Stem Cell” by Nissim Benvenisty is licensed under CC BY 2.5
There are many different applications and therapies of induced pluripotent stem cells. They are used in regenerative medicine for things such as neurodegeneration in diseases such as Alzheimer’s. They are used in disease modeling which allows for the form of the disease to be known and treated. They are also used in drug development and gene therapy as well as the production of patient specific cells that allow for elimination of immune rejection. The largest advantage of using induced pluripotent stem cells is that they are autologous cells, meaning that they come directly from the host so they will not be rejected. However, induced pluripotent stem cell therapy can be expensive.
Embryonic stem cell therapy occurs when a somatic cell nucleus is transferred from an individual enucleated oocyte which then undergoes embryonic development to the blastocyst stage. The cells can be expanded in culture and seeded directly onto a scaffold where differentiation can be induced. Embryonic stem cells can be used in many procedures including the replacement of dead nerve cells that occur due to Parkinson’s disease, replacement of dead heart muscle cells that are a result of heart attacks, replacement of degenerated skeletal muscle as a result of muscular dystrophy, and creation of insulin secreting cells for individuals suffering from Type 1 diabetes (Watt, Fiona, Driskell, 2010). They are used to replace or restore tissue damaged by disease or injury.
Progression of Stem Cell Medicine
Key Takeaway
Stem cells were discovered about 40 years ago in 1981 when Martin Evans discovered them at the University of Cambridge in mice embryos. Following this discovery, a large amount of research was done on the cells. This research led to the first artificially cloned animal, Dolly the sheep, in 1997. This led to the hope that one day human clones could be made through stem cells. Human embryonic stem cells were isolated in 1998 and grown in a lab for the first time ever. With the research of embryonic stem cells growing, the National Institutes of Health (NIH) released guidelines in 2000 that stated the rules in which the research had to fall under. However, federal funding for stem cell research was limited in 2001 by President Bush when a human embryonic stem cell was destroyed in a lab (Coghlan, 2014).
In 2005, Congress passed an act that would further stem cell research. However, it is vetoed by President Bush and the House could never get the ⅔ needed to override the veto. The first introduction to Embryonic stem cells came in 2006 when Shinya Yamanaka discovered a way to reprogram Adult stem cells to have embryonic like properties. The stem cell research limits paced by president Bush were lifted in 2009 when President Obama took over. The first injury ever treated through stem cell therapy was a spinal injury in 2010. Stem cell research funding was withheld in 2011 when the court ruled in favor of stem cell research in the case Sherley v. Sebelius. In 2012, stem cells were used in a study to treat medical blindness. Therapeutic cloning proved successful in Oregon in 2013 with human embryonic stem cells, and in 2014 it was successful through the use of adult stem cells. Induced Pluripotent stem cells were used in a trial to reduce age related blindness in 2014. President Obama signed the 21st Century Cures Act into law in 2016 which is meant to accelerate medical product development as well as innovation (“Timeline of Major Events in Stem Cell Therapy”,2016).
Timeline of Stem Cell Research
-
- 1981- Stem Cells found in mice embryos
- 1997- The first artificial animal clone is created through the use of stem cells
- 1998- Human embryonic stem cells are isolated and grown in a lab
- 2000- NIH guidelines are established
- 2001- Bush allows for limited embryonic research
- 2005- Congress passes stem cell research enhancement
- 2006- Induced pluripotent stem cells are created from adult cells
- 2009- Obama lifts restrictions from 2001 on stem cell research
- 2010- Spinal injury is treated through the use of stem cells, the first time they have ever been used to treat something
- 2011- Court rules in favor of Embryonic stem cell research in Sherley v. Sebelius
- 2012- Stem cells used in treatment that reduces blindness
- 2013- Therapeutic cloning is successful in a lab in Oregon
- 2014- Therapeutic cloning is successful with adult stem cells
- 2014- Trial for age related blindness treated through the use of iPSCs
- 2016- President Obama signs 21st Century Cures Act into law
Tissue Engineering with Stem Cells and its Application
Key Takeaway
Tissue engineering is the process of using a combination of scaffolds, biological materials (cells), and growth factors to replace damaged tissue. Stem cells and artificial materials are often used in this process to form necessary tissues a patient needs to survive (“Tissue Engineering and Regenerative Medicine”, n.d). The use of tissue engineering can prove highly beneficial in preventing rejection, or the immune response the body undergoes when forgien tissues or organs are used. This is due to the fact that the tissues that are introduced to the body are made from that same body’s cells. This process mainly focuses on the growth of musculoskeletal tissue, bone, and cartilage. Tissue engineering starts with a scaffold, or mold that is responsible for the structure of the new tissue. The scaffold has to be 3 dimensional, must be made of a material that does not interfere with cell growth, and be highly elastic. These factors are crucial in the process of tissue engineering, if one or more of these parameters are not met within the scaffold, tissue growth suitable for human transplant is improbable. Different materials for the scaffold are used depending on the type of tissue it calls for. For example, Poly L-lactide is used in oral surgery scaffolds, while polyglycolide is used primarily in orthopedic surgery scaffolds. The cells are then inserted into the scaffold and cultured in-order to differentiate the stem cells into their desired tissue. During this culturing process, changes in the environment might need to be made in order to properly create certain types of the tissue. Once the tissue is mature, it is ready for surgical transplantation. The current application of tissue engineering is limited to smaller projects such as veins, arteries, skin grafts, and trachea ( Hutmacher, 2000). As seen in Diagram 33.4 we can see a vascular graft made by the process of tissue engineering.
Vascular Grafts
Vascular Grafts are made for use in cardiovascular surgery in order to increase the rate of blood flow in arteries and veins. These grafts are often used in patients with plaque build up in their arteries, and prevent heart attack or other cardiovascular problems. Synthetic grafts have been known to cause infections, stenosis, thrombosis, or even calcification, all having very negative impacts on the body. With the implication of biocompatible grafts, made from tissue engineering, their risk has been minimized and their applications have been broadened. The main use of these new biocompatible grafts have been in pediatric surgery. Synthetic grafts have always had a downside in the pediatric field, they had limited growth potential after implantation. Because children’s bodies were always growing, a new solution was needed to fix this problem. Biocompatible grafts provide a solution, they have the ability to grow, self-repair, and regenerate, just like any normal human tissue (Matsuzaki et al. 2019).

“Gefäßprothese.JPG” by HIA is licensed under CC BY 3.0
Organogenesis with Stem Cells and its Applications
Key Takeaway
Through the process of organogenesis, medical professionals have been able to use stem cells to create human organs.
In another process called organogenesis, scientists have been able to create organs used in humans. This process refers to the growth of organs from a cell or group of cells. Organogenesis often starts with embryonic stem cells being implanted into a blastocyst, which is the stage of development in which we first see tissue differentiation, as seen in the photo below:
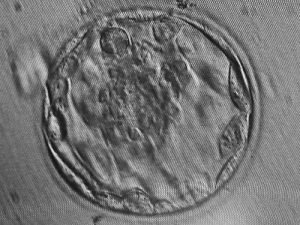
Blastocyst.JPG”by Harimiao is licensed under CC BY-SA 3
This encourages cell growth and division, and allows this newly formed structure to produce any organ found in the human body. While this sounds very useful, there are some faults with organogenesis: (1) Rejection of the new formed organ is likely, due to the fact that the stem cells being used are not derived from the patient. (2) The organ(s) produced are very small, almost relative to the size needed to function in a human fetus. In order to counter these inevitable consequences, organs produced by organogenesis are often implanted into other mammals to fully develop, often a pig. When introduced to a patient’s body, immunosuppressant drugs are used to reduce the chance of rejection. Since these treatments are relatively new, they must undergo rigorous animal testing in order for it to be considered for humans (Cascalho & Platt, 2006).
Animal Right Activist and Their View on Animal Testing
This sparks much uprising from animal rights groups, PETA, known to be one of these groups, has been at the forefront of the fight against animal cruelty and testing. They have made many strides in the fight agaisnt animal testing. For example on February 12, 2020 PETA was able to work with Suave to ban all animal testing for their products, and on May 12, 2020 they were able to help develop a COVID-19 ventilator without the use of animal testing (“Victories: Animal Experimentation”, 2020). These two instances prove society and science can still improve without the need for animal testing. While this is true, the US National Library of Medicine states that “… the total elimination of animal testing will significantly set back the development of essential medical devices, medicines, and treatment.”(Hajar, 2011). From this quote we can see how important animal testing is to the medical field. It is predicted, without a suitable, living, test subject medical professional can assess the side effects of certain treatments or medicines.
Stem Cells and Cancer
Key Takeaway
In an article published by American Cancer Society, they named cancer as the second leading cause of death in the United States ( Siegel et al., 2020). Due to cancer’s clear effect on the American population it is crucial that we understand what cancer is and how it directly impacts the human body. According to the National Cancer Institute, cancer is the name given to all diseases in which cells in the body undergo division without termination. These series of growths are initiated when abnormal or damaged cells fail to undergo apoptosis, programmed cell death, and the body starts creating new cells that are not needed. Cancer can occur in virtually any area of the body making it very hard to detect as well as treat. Cancerous growths can be very dangerous to the body that they are in, because they often impair functions of surrounding tissues and organs (“What is Cancer?”, n.d). According to Gary Larson, the medical director at the cancer institute in Oklahoma City, cancerous growths can have a wide array of effects on the human body depending on their location. For example if cancer occurs in leukemic cells (cells that make red and white blood cells, and platelets) fatal infections, bleeding, and oxygen loss can all occur (“What Makes Cancer so Lethal ?”, 2017).
The Stem Cell Theory of Cancer
Many theories about cancer have been proposed, but one theory sticks out due to its focus on the actual replication and growth of cancer cells. The stem cell theory of cancer states that cancerous growths are made of cancer stem cells (CSC) that are the driving force behind the division, and the overall lethality of tumors. These cells were first noticed when studying leukemia, and later identified in other types of cancers such as brain, colon, breast, liver, and ovarian. CSCs act almost identically to other types of stem cells, because they have the ability to self-renew and differentiate (Li et al., 2011; Buczacki, 2016). Diagram 33.6, illustrates this process. The red cells represent the CSCs and the white cells represent differentiated tumor cells. The CSCs will usually create a differentiated cell, while the others differentiated cell will divide creating a copy of themselves. This shows how fast tumors can grow and how one stem cell can create such massive tumors.
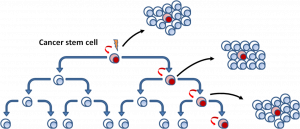
“Cancer stem cells model” by Malymajo is licensed under CC BY-SA 3.0
What do Cancer Stem Cells Mean for Cancer Treatments
Cancer treatments often fail due to the presence of CSC and the attributes they possess. They are often resistant to the conventional treatments which normally kill the already differentiated CSCs, therefore causing non-differentiated CSCs to remain and repopulate the cancerous growths (Li et al., 2011). One way to kill CSC could be chemotherapy. Chemotherapy is the use of powerful chemicals to kill rapidly dividing cancer cells. While this treatment has proven effective in some cases, some believe that chemotherapy is actually worse than the symptoms of certain cancers. According to the Mayo Clinic, chemotherapy is known to cause heart problems, nerve and lung damage, infertility, and even poses the risk of a second cancer. These side effects are mostly due to the fact that drugs used in this type of therapy target rapidly dividing cells indiscriminately, therefore causing damage to non-cancerous cells (“Chemotherapy”. n.d). Due to these costly side effects, scientists turn to other methods to kill CSCs. It was found that growth of stem cells are regulated by different signaling pathways, and it was theorized that by blocking these pathways in CSCs, tumor growth would cease. Many naturally occurring compounds found in common food items are known to specifically block some of the pathways that contribute to specifically CSC division. Turmeric, soy, grapes, tomatoes, fish, egg yolks, plums, peanuts, and papayas all contain these types of compounds that have been clinically proven to be able to block certain signalling pathways found in CSCs ( Li et al., 2011). While this is true, many discoveries still need to be made in order to fully understand cancer and how to cure it.
Chapter Summary
- Stem cell therapies can be formed from three different types of cells: Adult stem cells, induced pluripotent stem cells, and embryonic stem cells
- Over the last 20-40 years there has been an increase in the amount of testing of stem cells along with a increases amount of application in the medical field when it comes to treating diseases and injuries
- The application of stem cells to tissue engineering has allowed treatments to be more personalized to the patient and allow for the growth of necessary bodily tissues
- Through the use of stem cells and blastocysts, scientists have been able to induce organogenesis and create organs needed for transplant.
- Stem cells have given insight to how cancer spreads and grows, allowing scientists to learn more about it and possibly develop new treatments.
Exercises
1) What kind of treatments can Embryonic stem cells be used for?
a) Parkinson’s Disease
b) Heart Attack
c) Muscular Dystrophy
d) All of the above
(Answer I all the above)
2) What year did President Bush limit funding for stem cell research?
a) 2009
b) 2010
c) 2001
d) 2007
(Answer is 2001)
3) In what stage of human development does a stem cell need to be implanted in order for organogenesis to occur ?
a) Blastomere
b) Blastocyst
c) Blastula
d) Blastocoel
( Answer is Blastocyst)
4) What is the name of the theory that suggests that certain types of stem cells are behind the growth of tumors ?
a) The molecular theory of cancer
b) The integrative theory of cancer
c) The stem cell theory of cancer
d) None of the Above
( Answer is the stem cell theory of cancer)
5) Name the three criteria that must be met when creating a scaffold for tissue engineering. Then describe the next steps taken to create a tissue.
(Answer should be similar to the following:
A scaffold must be 3 dimensional in shape, highly elastic, and be made of material that does not prevent cell growth. After a scaffold is made, biological material is then inserted into the mold. The mold is then cultured in a specific environment unique to the certain tissue being made. The final tissue is finally edited to specifically fit the needs of the patients and then implanted.)
End of Chapter Summary :
References
Bernard Lo, Lindsay Parham, Ethical Issues in Stem Cell Research, Endocrine Reviews, Volume 30, Issue 3, 1 May 2009, Pages 204–213, https://doi.org/10.1210/er.2008-0031
Buczacki, S. (2016). Cancer Stem Cell. Cancer Stem Cell – an overview . https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/cancer-stem-cell.
Cascalho, M., & Platt, J. L. (2006, March). The future of organ replacement: needs, potential applications, and obstacles to application. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475508/.
Coghlan, A. (2014, January 30). Stem cell timeline: The history of a medical sensation. Retrieved November 17, 2020, from https://www.newscientist.com/article/dn24970-stem-cell-timeline-the-history-of-a-medical-sensation/
Hajar, R. (2011, January). Animal testing and medicine. Heart views : the official journal of the Gulf Heart Association. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3123518/.
Hutmacher, D. W. (200AD, December 15). Scaffolds in tissue engineering bone and cartilage. Clemson University Libraries – Login. https://www-sciencedirect-com.libproxy.clemson.edu/science/article/pii/S0142961200001216?via=ihub.
Kim, S., & Vellis, J. (2009, March 19). Stem cell‐based cell therapy in neurological diseases: A review. Retrieved November 17, 2020, from https://onlinelibrary.wiley.com/doi/full/10.1002/jnr.22054
Li, Y., Wicha, M. S., Schwartz, S. J., & Sun, D. (2011, February 4). Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. The Journal of Nutritional Biochemistry. https://www.sciencedirect.com/science/article/pii/S0955286310002445.
Lindvall, Olle, and Insoo Hyun. “Medical Innovation versus Stem Cell Tourism.” Science, vol. 324, no. 5935, 2009, pp. 1664–1665. JSTOR, www.jstor.org/stable/20536494. Accessed 17 Nov. 2020.
Matsuzaki, Y., John, K., Shoji, T., & Shinoka, T. (2019, March 27). The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6937136/.
Mayo Foundation for Medical Education and Research. (2020, March 5). Chemotherapy. Mayo Clinic. https://www.mayoclinic.org/tests-procedures/chemotherapy/about/pac-20385033.
PETA. Victories: Animal Experimentation. PETA. https://www.peta.org/category/main-issues/animal-experimentation/?post_type=victory.
Quora. (2017, March 7). What Makes Cancers So Lethal? https://www.forbes.com/sites/quora/2017/03/07/what-makes-cancers-so-lethal/?sh=21056a15300f.
Scadden, David T. “Stem Cell-Based Medicine.” Proceedings of the American Philosophical Society, vol. 153, no. 2, 2009, pp. 185–192. JSTOR, www.jstor.org/stable/40541657. Accessed 17 Nov. 2020.
Segers, V., Lee, R. Stem-cell therapy for cardiac disease. Nature 451, 937–942 (2008). https://doi.org/10.1038/nature06800
Siegel, R. L., Miller, K. D., & Jemal, A. (2020, January 8). Cancer statistics, 2020. American Cancer Society Journals. https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21590.
Timeline of major events in stem cell research policy. (2016, December 30). Retrieved November 17, 2020, from https://www.researchamerica.org/advocacy-action/issues-researchamerica-advocates/stem-cell-research/timeline-major-events-stem-cell
U.S. Department of Health and Human Services. Tissue Engineering and Regenerative Medicine. National Institute of Biomedical Imaging and Bioengineering. https://www.nibib.nih.gov/science-education/science-topics/tissue-engineering-and-regenerative-medicine.
Watt, Fiona M, and Ryan R Driskell. “The therapeutic potential of stem cells.” Philosophical transactions of the Royal Society of London. Series B, Biological sciences vol. 365,1537 (2010): 155-63. doi:10.1098/rstb.2009.0149
Wei, X., Yang, X., Han, Z. et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34, 747–754 (2013). https://doi.org/10.1038/aps.2013.50
What Is Cancer? National Cancer Institute. https://www.cancer.gov/about-cancer/understanding/what-is-cancer
Yu, J., Vodyanik, M., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J., Tian, S., . . . Thomson, J. (2007, December 21). Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Retrieved November 17, 2020, from https://science.sciencemag.org/content/318/5858/1917/tab-pdf
