Learning Objectives
By the end of this section, you will be able to:
- Identify the three basic parts of a measured quantity
- Describe the properties and units of length, mass, volume, density, temperature, and time
- Convert between various metric units
- Calculate density using appropriate units
Our ability to make quantitative observations is one of the cornerstones of the scientific method. In chemistry we commonly measure quantities by mass and volume. We also frequently work with density and concentration, and a wide assortment of other quantitative measurements and descriptions. Measurements provide the macroscopic information that is the basis of most of the hypotheses, theories, and laws that describe the behavior of matter and energy in both the macroscopic and microscopic domains of chemistry.
Every measurement provides three kinds of information: a number (quantitative observation), a unit (describes how it was measured), and the degree of reliability (uncertainty of the measurement). While the number and unit are explicitly represented when a quantity is written, the uncertainty is an aspect of the measurement result that is more implicitly represented and will be discussed later.
The number in the measurement can be represented in different ways, including decimal form and scientific notation. For instance, the mass of the average mosquito is about 0.0000025 kilograms, which can be written as 2.5 × 10−6 kg.
Units, such as liters, pounds, and centimeters, are standards of comparison for measurements. When we buy a 2-liter bottle of a soft drink, we expect that the volume of the drink was measured, so it is two times larger than the volume that everyone agrees to be 1 liter. The meat used to prepare a 0.25-pound hamburger is measured so it weighs one-fourth as much as 1 pound. Without units, a number can be meaningless, confusing, or possibly life threatening if it refers, for instance, to the dose of a necessary medicine.
Scientific measurements are often expressed in SI units, the modern version of the metric system, as listed in Table 1. Other units can be derived from these base units. The standards for these units are fixed by international agreement, and they are called the International System of Units or SI Units (from the French, Le Système International d’Unités).
| Property Measured | Name of Unit | Symbol of Unit |
|---|---|---|
| length | meter | m |
| mass | kilogram | kg |
| time | second | s |
| temperature | kelvin | K |
| electric current | ampere | A |
| amount of substance | mole | mol |
| luminous intensity | candela | cd |
| Table 1. Base Units of the SI System | ||
Very commonly we use units that are fractions or multiples of a base unit, based on powers of 10. Fractional or multiple SI units are named using a prefix and the name of the base unit. For example, a length of 1000 meters is also called a kilometer because the prefix kilo means “one thousand,” which in scientific notation is 103 (1 kilometer = 1000 m = 103 m). The prefixes used and the powers to which 10 are raised are listed in Table 2.

Need a refresher or more practice with scientific notation? Visit this site to go over the basics of scientific notation.
| Prefix | Symbol | Factor | Example |
|---|---|---|---|
| femto | f | 10−15 | 1 femtosecond (fs) = 1 × 10−15 s (0.000000000000001 s) |
| pico | p | 10−12 | 1 picometer (pm) = 1 × 10−12 m (0.000000000001 m) |
| nano | n | 10−9 | 4 nanograms (ng) = 4 × 10−9 g (0.000000004 g) |
| micro | µ | 10−6 | 1 microliter (μL) = 1 × 10−6 L (0.000001 L) |
| milli | m | 10−3 | 2 millimoles (mmol) = 2 × 10−3 mol (0.002 mol) |
| centi | c | 10−2 | 7 centimeters (cm) = 7 × 10−2 m (0.07 m) |
| deci | d | 10−1 | 1 deciliter (dL) = 1 × 10−1 L (0.1 L ) |
| kilo | k | 103 | 1 kilometer (km) = 1 × 103 m (1000 m) |
| mega | M | 106 | 3 megahertz (MHz) = 3 × 106 Hz (3,000,000 Hz) |
| giga | G | 109 | 8 gigayears (Gyr) = 8 × 109 yr (8,000,000,000 Gyr) |
| tera | T | 1012 | 5 terawatts (TW) = 5 × 1012 W (5,000,000,000,000 W) |
| Table 2. Common Unit Prefixes | |||
SI Base Units
Length
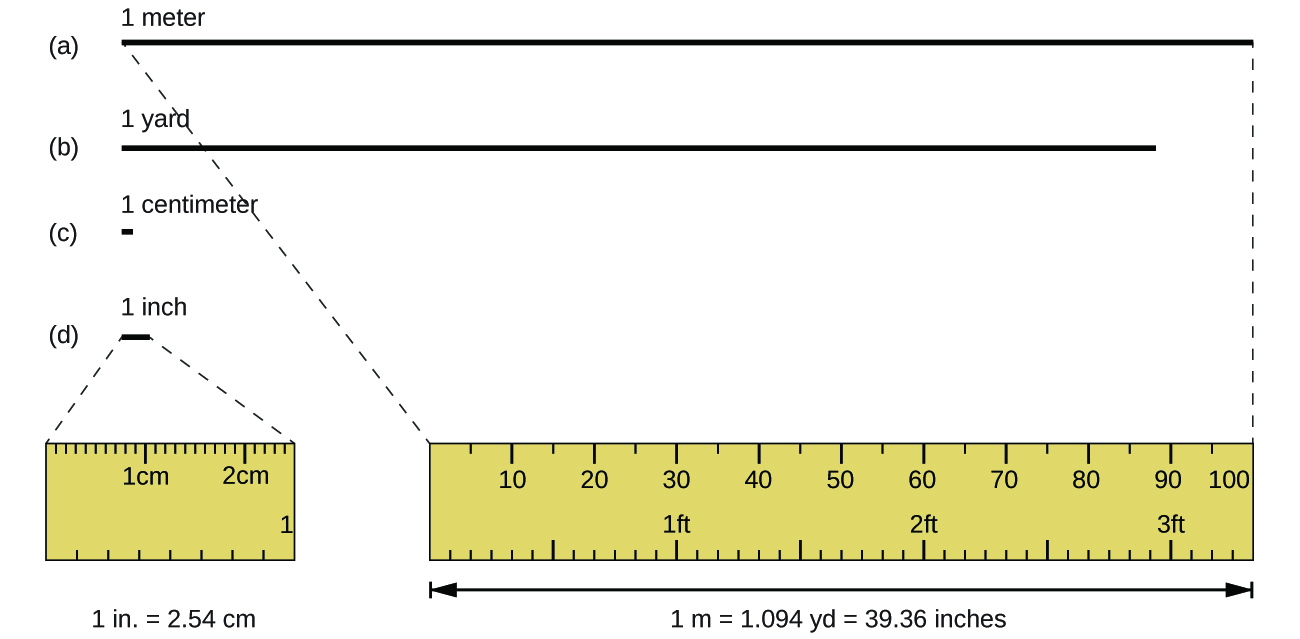
The standard unit of length in both the SI and original metric systems is the meter (m). A meter is about 3 inches longer than a yard (Figure 1). Longer distances are often reported in kilometers (1 km = 1000 m = 103 m), whereas shorter distances can be reported in centimeters (1 cm = 0.01 m = 10−2 m) or millimeters (1 mm = 0.001 m = 10−3 m).
Mass
The standard unit of mass in the SI system is the kilogram (kg). A kilogram was originally defined as the mass of a liter of water (a cube of water with an edge length of exactly 0.1 meter). This mass standard is now defined in terms of the second and the metre, based on fixed fundamental constants of nature. One kilogram is about 2.2 pounds. The gram (g) is exactly equal to 1/1000 of the mass of the kilogram (10−3 kg).

Temperature
The SI unit of temperature is the kelvin (K). The convention is to use kelvin (all lowercase) for the word, K (uppercase) for the unit symbol, and neither the word “degree” nor the degree symbol (°). The degree Celsius (°C) is also allowed in the SI system, with both the word “degree” and the degree symbol used for Celsius measurements. Celsius degrees are the same magnitude (size) as those of kelvin, but the two scales place their zeros in different places. Water freezes at 273.15 K (0 °C) and boils at 373.15 K (100 °C) by definition, and normal human body temperature is approximately 310 K (37 °C).
Time
The SI base unit of time is the second (s). Small and large time intervals can be expressed with the appropriate prefixes; for example, 3 microseconds = 0.000003 s = 3 × 10−6 and 5 megaseconds = 5,000,000 s = 5 × 106 s. Alternatively, hours, days, and years can be used.
Derived Units
We can derive many units from the seven SI base units. For example, we can use the base unit of length to define a unit of volume, and the base units of mass and volume to define a unit of density.
Volume
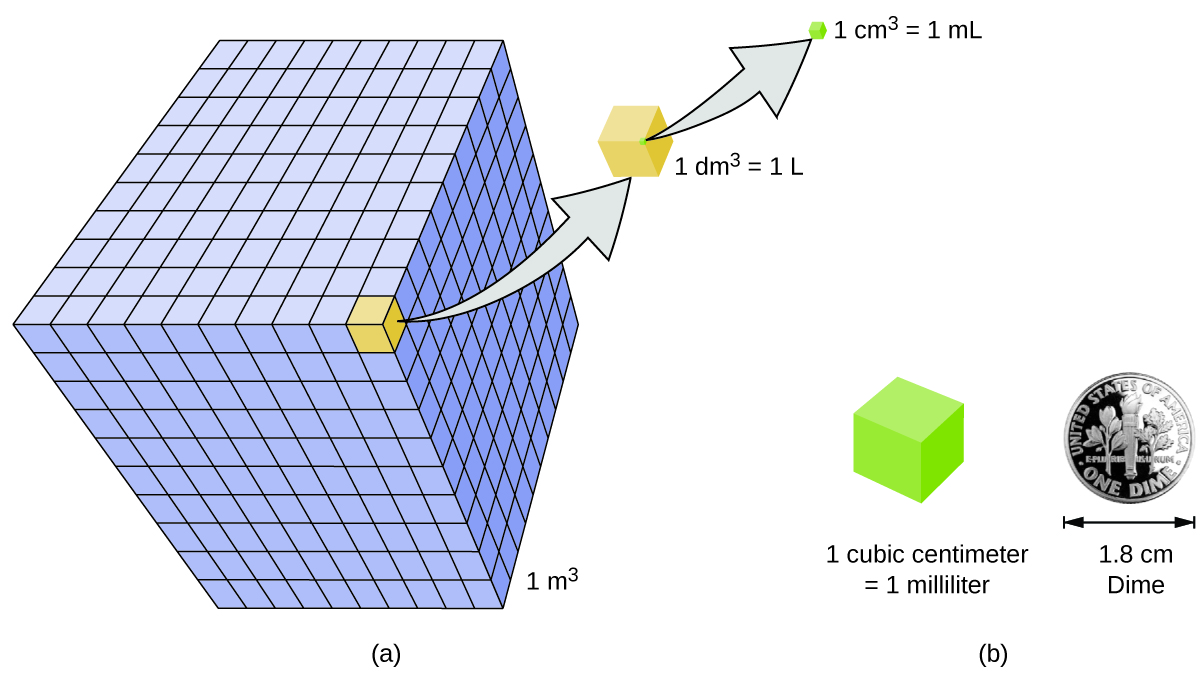
Volume is the measure of the amount of space occupied by an object. The standard SI unit of volume is defined by the base unit of length (Figure 3). The standard volume is a cubic meter (m3), a cube with an edge length of exactly one meter.
A more commonly used unit of volume is the liter (L). A cube with edge lengths of exactly one decimeter (0.1 m, or 10 cm) contains this volume.
A cubic centimeter is also called a milliliter (mL) and is 1/1000 of a liter. A mL is equivalent to one cubic centimeter (cm3). The abbreviation cc (for cubic centimeter) is used in medical settings.
Density
We use the mass and volume of a substance to determine its density.
The density of a substance is the ratio of the mass of a sample of the substance to its volume. For many situations. the SI unit definition (kg/m3) for density is an inconvenient unit, so grams per cubic centimeter (g/cm3 or g/mL) is often used for the densities of solids and liquids, and grams per liter (g/L) for gases.
| Solids | Liquids | Gases (at 25 °C and 1 atm) |
|---|---|---|
| ice (at 0 °C) 0.92 g/cm3 | water 1.0 g/cm3 | dry air 1.20 g/L |
| oak (wood) 0.60–0.90 g/cm3 | ethanol 0.79 g/cm3 | oxygen 1.31 g/L |
| iron 7.9 g/cm3 | acetone 0.79 g/cm3 | nitrogen 1.14 g/L |
| copper 9.0 g/cm3 | glycerin 1.26 g/cm3 | carbon dioxide 1.80 g/L |
| lead 11.3 g/cm3 | olive oil 0.92 g/cm3 | helium 0.16 g/L |
| silver 10.5 g/cm3 | gasoline 0.70–0.77 g/cm3 | neon 0.83 g/L |
| gold 19.3 g/cm3 | mercury 13.6 g/cm3 | radon 9.1 g/L |
| Table 3. Densities of Common Substances | ||
While there are many ways to experimentally determine the density of an object, perhaps the most straightforward method involves separately finding the mass and volume of the object, and then dividing the mass of the sample by its volume.
In the following example, the mass is found directly by weighing, but the volume is found indirectly through length measurements.
Example 1
Gold—in bricks, bars, and coins—has been a form of currency for centuries. In order to swindle people into paying for a brick of gold without actually investing in a brick of gold, people have considered filling the centers of hollow gold bricks with lead to fool buyers into thinking that the entire brick is gold. It does not work: Lead is a dense substance, but its density is not as great as that of gold, 19.3 g/cm3. What is the density of lead if a cube of lead has an edge length of 2.00 cm and a mass of 90.7 g?
Solution
The density of a substance can be calculated by dividing its mass by its volume. The volume of a cube is calculated by cubing the edge length.
[latex]\text{volume of lead cube}=2.00\text{cm}\times2.00\text{cm}\times2.00\text{cm}=9.00\text{cm}^3[/latex]
[latex]\text{density}=\frac{\text{mass}}{\text{volume}}=\frac{90.7\text{g}}{8.00\text{cm}^3}=\frac{11.3\text{g}}{1.00\text{cm}^3}=11.3\;\text{g}/\text{cm}^3[/latex]
(We will discuss the reason for rounding to the first decimal place in the next section.)

To learn more about the relationship between mass, volume, and density, use this interactive simulator to explore the density of different materials, like wood, ice, brick, and aluminum.
Example 2
This PhET simulation illustrates another way to determine density, using displacement of water. Determine the density of the red and yellow blocks.
Solution
When you open the density simulation and select Same Mass, you can choose from several 5.00-kg colored blocks that you can drop into a tank containing 100.00 L water. The yellow block floats (it is less dense than water), and the water level rises to 105.00 L. While floating, the yellow block displaces 5.00 L water, an amount equal to the weight of the block. The red block sinks (it is more dense than water, which has density = 1.00 kg/L), and the water level rises to 101.25 L.
The red block therefore displaces 1.25 L water, an amount equal to the volume of the block. The density of the red block is:
Note that since the yellow block is not completely submerged, you cannot determine its density from this information. But if you hold the yellow block on the bottom of the tank, the water level rises to 110.00 L, which means that it now displaces 10.00 L water, and its density can be found:
[latex]\text{density}=\frac{\text{mass}}{\text{volume}}=\frac{\text{5.00 kg}}{\text{10.00 L}}=0.500 \text{kg/L}[/latex]
Test Yourself
Remove all of the blocks from the water and add the green block to the tank of water, placing it approximately in the middle of the tank. Determine the density of the green block.
Answer
2.00 kg/L
The Angstrom Unit
Although not an SI unit, the angstrom (Å) is a useful unit of length. It is one ten-billionth of a meter, or 10−10 m. Why is it a useful unit? The ultimate particles that compose all matter are about 10−10 m in size, or about 1 Å. This makes the angstrom a natural—though not approved—unit for describing these particles.
The angstrom unit is named after Anders Jonas Ångström, a nineteenth-century Swedish physicist. Ångström’s research dealt with light being emitted by glowing objects, including the sun. Ångström studied the brightness of the different colors of light that the sun emitted and was able to deduce that the sun is composed of the same kinds of matter that are present on the earth. By extension, we now know that all matter throughout the universe is similar to the matter that exists on our own planet.
Anders Jonas Ångstrom, a Swedish physicist, studied the light coming from the sun. His contributions to science were sufficient to have a tiny unit of length named after him, the angstrom, which is one ten-billionth of a meter.
Source: Photo of the sun courtesy of NASA’s Solar Dynamics Observatory, http://commons.wikimedia.org/wiki/File:The_Sun_by_the_Atmospheric_Imaging_Assembly_of_NASA%27s_Solar_Dynamics_Observatory_-_20100801.jpg.
Key Concepts and Summary
Measurements provide quantitative information that is critical in studying and practicing chemistry. Each measurement has an amount, a unit for comparison, and an uncertainty. Measurements can be represented in either decimal or scientific notation. Scientists primarily use the SI (International System) or metric systems. We use base SI units such as meters, seconds, and kilograms, as well as derived units, such as liters (for volume) and g/cm3 (for density). In many cases, we find it convenient to use unit prefixes that yield fractional and multiple units, such as microseconds (10−6 seconds) and megahertz (106 hertz), respectively.
Key Equations
- [latex]\text{density}=\frac{\text{mass}}{\text{volume}}[/latex]
Review-Reflect, Extend
Review-Reflect
1. Identify the unit in each quantity.
a) 2 boxes of crayons b) 3.5 grams of gold
2. Indicate what multiplier each prefix represents.
a) c b) G c) μ
3. Give the prefix that represents each multiplier.
a) 1/1,000th × b) 1,000 × c) 1,000,000,000 ×
4. Give the prefix that represents each multiplier.
a) 1/1,000,000,000th × b) 1/100th × c) 1,000,000 ×
5. Express each quantity in a more appropriate unit. There may be more than one acceptable answer.
a) 43,600 mL b) 0.0000044 m c) 1,438 ms
6. Indicate the SI base units or derived units that are appropriate for the following measurements:
b) the distance from Dallas to Oklahoma City
e) the temperature at which alcohol boils
g) the volume of a flu shot or a measles vaccination
Extend
1. You may have heard the terms microscale or nanoscale to represent the sizes of small objects. What units of length do you think are useful at these scales? What fractions of the fundamental unit of length are these units?
2. Acceleration is defined as a change in velocity per time. Propose a unit for acceleration in terms of the fundamental SI units.
3. Visit this PhET density simulation and select Mystery Blocks.
a) Pick one of the Mystery Blocks and determine its mass, volume, density, and its likely identity.
b) Pick a different Mystery Block and determine its mass, volume, density, and its likely identity.
c) Order the Mystery Blocks from least dense to most dense. Explain.
Answers
1. a) boxes of crayons b) grams of gold
2. a) 1/100 x b) 1,000,000,000 x c) 1/1,000,000 x
3. a) milli- b) kilo- c) giga-
4. a) nano- b) centi- c) mega-
5. a) 43.6 L b) 4.4 µm c) 1.438 s
6. a) kilograms b) meters c) kilometers/second d) kilograms/cubic meter e) kelvin f) square meters g) cubic meters
Extend problem from PhET:
3. a) and b) answer is one of the following. A/yellow: mass = 65.14 kg, volume = 3.38 L, density = 19.3 kg/L, likely identity = gold. B/blue: mass = 0.64 kg, volume = 1.00 L, density = 0.64 kg/L, likely identity = apple. C/green: mass = 4.08 kg, volume = 5.83 L, density = 0.700 kg/L, likely identity = gasoline. D/red: mass = 3.10 kg, volume = 3.38 L, density = 0.920 kg/L, likely identity = ice; and E/purple: mass = 3.53 kg, volume = 1.00 L, density = 3.53 kg/L, likely identity = diamond. (c) B/blue/apple (0.64 kg/L) < C/green/gasoline (0.700 kg/L) < C/green/ice (0.920 kg/L) < D/red/diamond (3.53 kg/L) < A/yellow/gold (19.3 kg/L)
Glossary
Celsius (°C): unit of temperature; water freezes at 0 °C and boils at 100 °C on this scale
convention: a standard agreed upon by parties using a symbol or system
cubic centimeter (cm3 or cc): volume of a cube with an edge length of exactly 1 cm
cubic meter (m3): SI unit of volume
density: ratio of mass to volume for a substance or object
kelvin (K): SI unit of temperature; 273.15 K = 0 ºC
kilogram (kg): standard SI unit of mass; 1 kg = approximately 2.2 pounds
length: measure of one dimension of an object
liter (L): (also, cubic decimeter) unit of volume; 1 L = 1,000 cm3
meter (m): standard metric and SI unit of length; 1 m = approximately 1.094 yards
milliliter (mL): 1/1,000 of a liter; equal to 1 cm3
second (s): SI unit of time
SI units (International System of Units): standards fixed by international agreement in the International System of Units (Le Système International d’Unités)
unit: standard of comparison for measurements
volume: amount of space occupied by an object

