Learning Objectives
By the end of this section, you will be able to:
- Explain the organization of elements in the periodic table
- Predict some general properties of elements based on their location within the periodic table
There are many known elements, both naturally occurring and manmade. In ancient times the known elements were carbon, iron, sulfur, gold, silver, copper, tin, lead, mercury and zinc. It was not until the late 1700s that new elements began to be discovered (Ti, Zr, U, Te, Sr, Ce, Cr, Si, Se, Ce, Li, V, Th). In the 1800s Sir Humphrey Davy discovered several alkali and alkaline earth metals and halogens through the use of electricity. The development of spectroscopy spurred discovery of additional new elements in the 1800s, including discovery of Cesium, Rubidium, and Helium. Helium was first discovered by analyzing light from the sun in 1868 before it was found on Earth in 1882, through the spectral analysis of lava from Mount Vesuvius.
Spectroscopy remains a cornerstone of chemical analysis.
As early chemists worked to purify ores and discovered more elements, patterns in the chemical behavior of elements emerged. In 1789, Antoine Lavoisier arranged the 33 known chemical elements into four groups: gases, metals, nonmetals and earths. In 1829, Johann Wolfgang Döbereiner observed that many of the elements could be arranged in groups of three, now known as “Döbereiner’s triads”. One such grouping includes lithium (Li), sodium (Na), and potassium (K): These elements all are shiny, conduct heat and electricity well, and have similar chemical properties. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) also exhibit similar properties to each other, but these properties are drastically different from those of any of the elements above.
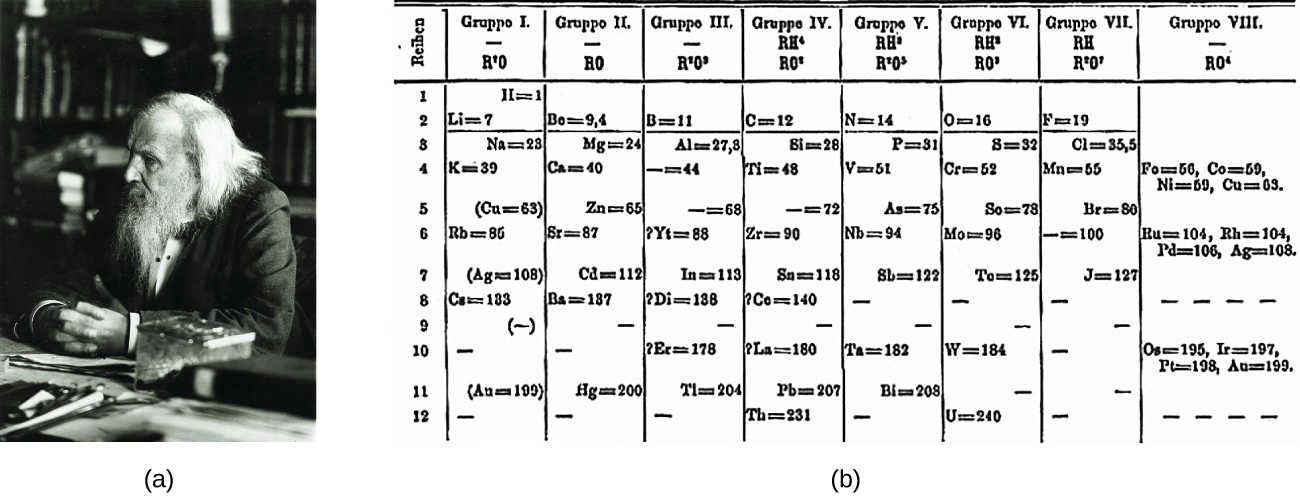
Dimitri Mendeleev in Russia (1869) and Lothar Meyer in Germany (1870) independently recognized that there was a periodic relationship among the properties of the elements known at that time. Both published tables with the elements arranged according to increasing atomic mass. But Mendeleev went one step further than Meyer: He used his table to predict the existence of elements that would have the properties similar to aluminum and silicon, but were yet unknown. The discoveries of gallium (1875) and germanium (1886) provided great support for Mendeleev’s work. Although Mendeleev and Meyer had a long dispute over priority, Mendeleev’s contributions to the development of the periodic table are now more widely recognized (Figure 1).
Once the structural features of atoms were understood, it became apparent that the periodic relationship involved these features. A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal rows, and 18 vertical groups. For the table to fit on a single page, parts of two of the rows, a total of 14 columns, are usually moved to a position below the main body of the table.
Many elements differ dramatically in their chemical and physical properties, but some elements are similar in their behaviors. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and conduct heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into large classes with common properties: metals (elements that are shiny, malleable, good conductors of heat and electricity—shaded yellow); nonmetals (elements that appear dull, poor conductors of heat and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded purple).
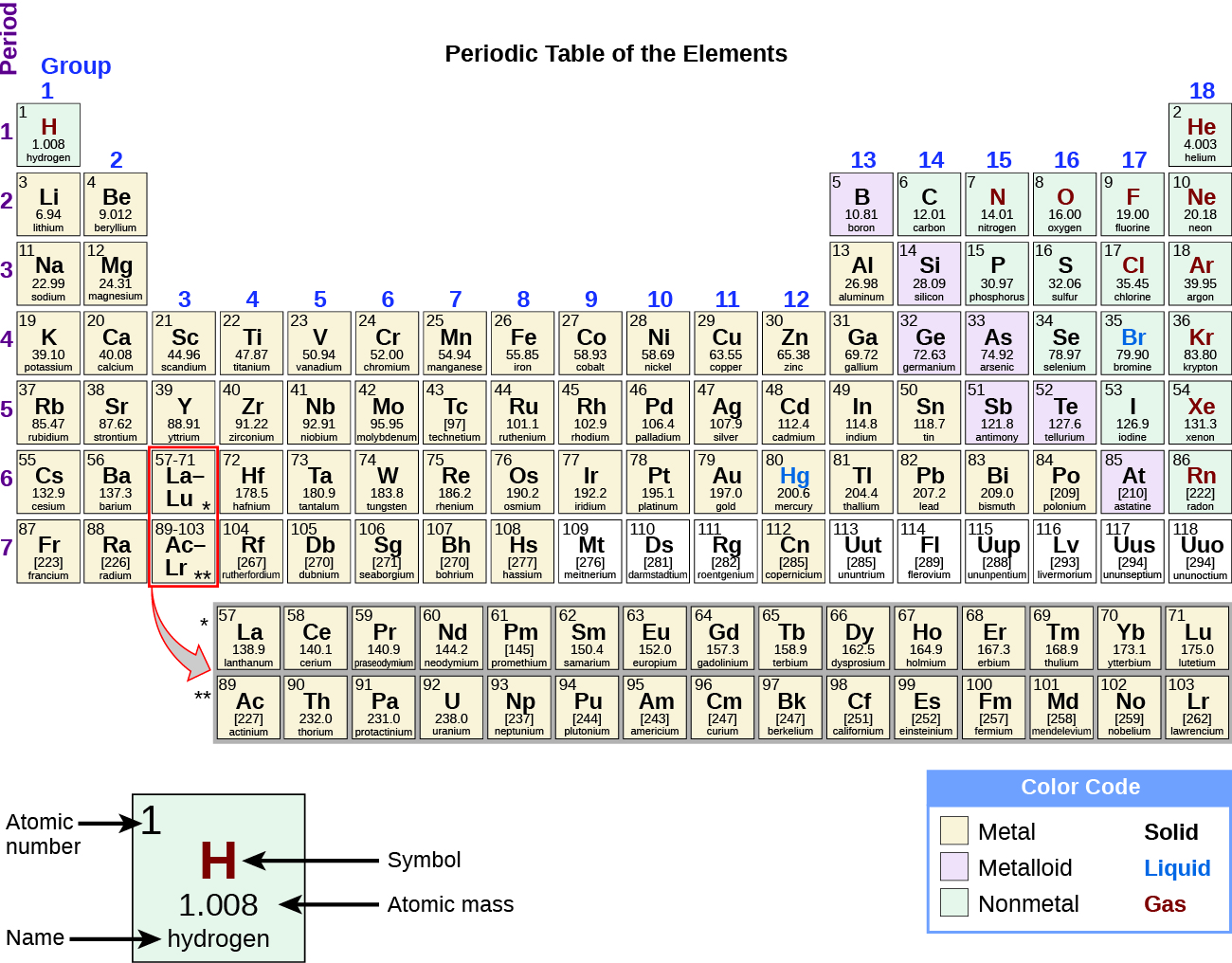
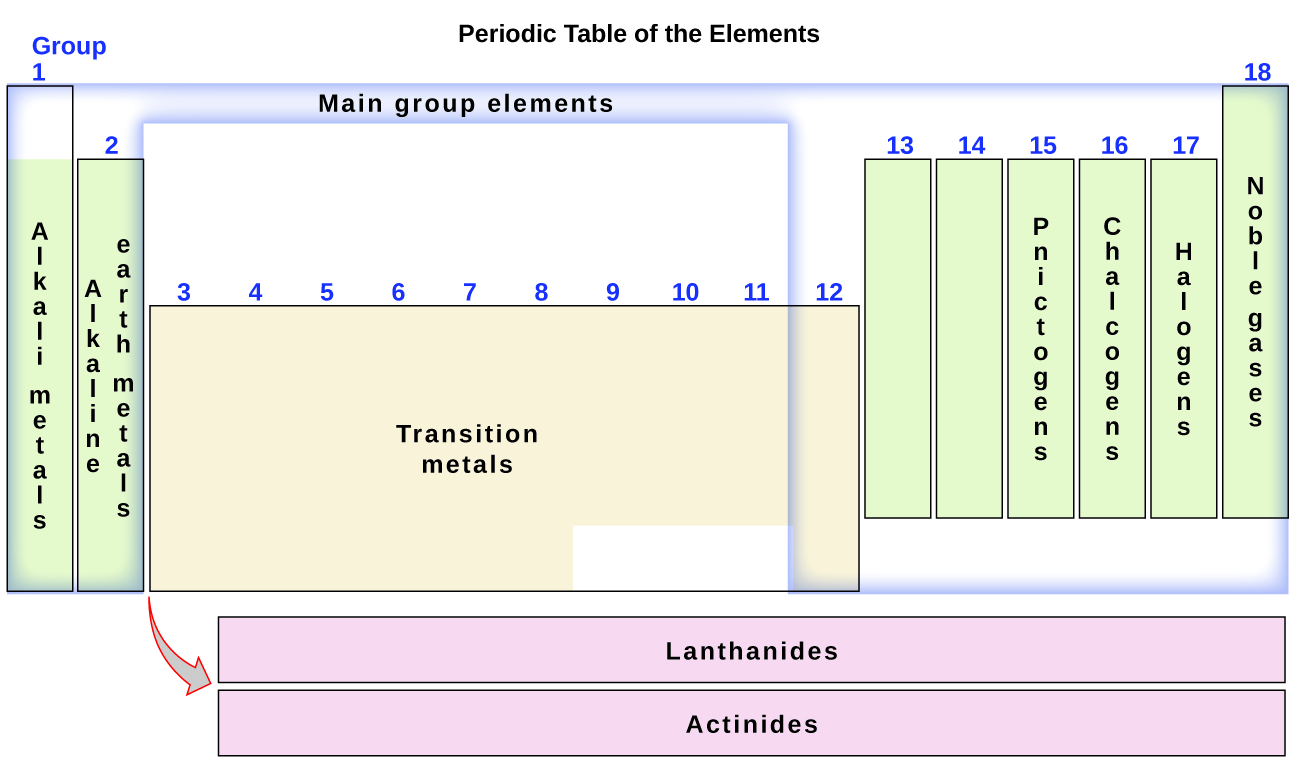
The elements can also be classified into the main-group elements (or representative elements) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12. The elements can be subdivided further by more specific properties, such as the composition of the compounds they form. For example, the elements in group 1 (the first column) form compounds that consist of one atom of the element and one atom of hydrogen. These elements (except hydrogen) are known as alkali metals, and they all have similar chemical properties. The elements in group 2 (the second column) form compounds consisting of one atom of the element and two atoms of hydrogen: These are called alkaline earth metals, with similar properties among members of that group. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (group 18, also known as inert gases). Hydrogen is a unique, nonmetallic element with properties similar to both group 1 and group 17 elements. For that reason, hydrogen sometimes is shown at the top of both groups, and sometimes sits by itself.
Most metals occur as solids. An exception to this is mercury (Hg) which occurs as liquid. Noble gases, in the far right column, occur naturally as gas. Many non-metals occur as multi-atomic molecules: (i.e. more than one atom together is the natural state): H2, O2, N2, F2, Cl2 which are all gases, S8, P4, Se8, I2 which are all solids and Br2 which is a liquid.

Click on this link for an interactive periodic table, which you can use to explore the properties of the elements (includes podcasts and videos of each element). You may also want to try this one that shows photos of all the elements.
Example 1
For the following elements, list their symbol, their natural state, and classify them as metal, nonmetal or metalloid:
a) magnesium b) silver c) uranium d) chlorine
Solution
a) Magnesium = Mg, occurs as a solid, is a metal (main group metal)
b) Silver = Ag, occurs as a solid, is a metal (transition metal)
c) Uranium = U, occurs as a solid, is a metal (inner transition metal)
d) Chlorine = Cl, occurs as Cl2in the gas state, is a nonmetal
In studying the periodic table, you might have noticed that some elements have their atomic mass given in square brackets. This is done for elements that consist entirely of unstable, radioactive isotopes. An average atomic weight cannot be determined for these elements because their radioisotopes may vary significantly in relative abundance, depending on the source, or may not even exist in nature. The number in square brackets is the atomic mass number (and approximate atomic mass) of the most stable isotope of that element.
Periodic tables also can be found with slightly differing average atomic masses for particular elements. This can be due to variations in isotopic abundances around the world; as a new source is found, abundance calculations can be shifted if the new supply has a non-matching isotopic distribution.
Key Concepts and Summary
The discovery of the periodic recurrence of similar properties among the elements led to the formulation of the periodic table, in which the relationships between elements are laid out in a graphical format. Elements in the same group of the periodic table have similar chemical properties. Elements can be classified in a variety of ways, including the large groups named metals, nonmetals, and metalloids.
Review-Refresh, Extend
Review-Refresh
1. Using the periodic table, classify each of the following elements as a metal or a nonmetal, and then further classify each as a main-group (representative) element or transition metal
a) bromine b) strontium c) neon
d) gold e) rhodium f) sulfur
g) carbon h) potassium
2. Identify the group and row numbers for each of the elements:
a) uranium b) bromine c) strontium d) neon
e) gold g) rhodium h) sulfur
Extend
- A fellow student says casually to you, “Why don’t they just keep it simpler by making the periodic table a rectangle?” Craft an answer to them that they will understand, even if they are not in chemistry class.
Answers to Review-Refresh
1. a) nonmetal, representative element b) metal, representative element; c) nonmetal, representative element; d) metal, transition metal; e) metal, transition metal; f) nonmetal, representative element; g) nonmetal, representative element; h) metal, representative element
2.
a) Bromine Row 4 Group 17 b) Strontium Row 5 Group 2 c) Neon Row 2 Group 18
d) Gold Row 6 Group 11 e) Rhodium Row 5 Group 9 f) Sulfur Row 3 Group 16
Glossary
actinide: inner transition metal in the bottom of the bottom two rows of the periodic table
alkali metal: element in group 1
alkaline earth metal: element in group 2
chalcogen: element in group 16
group: vertical column of the periodic table
halogen: element in group 17
inert gas: (also, noble gas) element in group 18
inner transition metal: (also, lanthanide or actinide) element in the bottom two rows; if in the first row, also called lanthanide, or if in the second row, also called actinide
lanthanide: inner transition metal in the top of the bottom two rows of the periodic table
main-group element: (also, representative element) element in columns 1, 2, and 12–18
metal: element that is shiny, malleable, good conductor of heat and electricity
metalloid: element that conducts heat and electricity moderately well, and possesses some properties of metals and some properties of nonmetals
noble gas: (also, inert gas) element in group 18
nonmetal: element that appears dull, poor conductor of heat and electricity
period: (also, series) horizontal row of the periodic table
periodic law: properties of the elements are periodic function of their atomic numbers.
periodic table: table of the elements that places elements with similar chemical properties close together
pnictogen: element in group 15
representative element: (also, main-group element) element in columns 1, 2, and 12–18
series: (also, period) horizontal row of the period table
transition metal: element in columns 3–11
