Learning Objectives
By the end of this module, you will be able to:
- Derive names for common types of inorganic compounds and simple molecular compounds using a systematic approach
Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids – subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry.
Nomenclature of Ionic Compounds
To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? Are the ions monatomic or polyatomic? If the compound is molecular, does it contain hydrogen? If so, does it also contain oxygen? From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
Compounds Containing Only Monatomic Ions
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix –ide). Some examples are given in Table 1.
| NaCl, sodium chloride | Na2O, sodium oxide |
| KBr, potassium bromide | CdS, cadmium sulfide |
| CaI2, calcium iodide | Mg3N2, magnesium nitride |
| CsF, cesium fluoride | Ca3P2, calcium phosphide |
| LiCl, lithium chloride | Al4C3, aluminum carbide |
| Table 1. Names of Some Ionic Compounds | |
Compounds Containing Polyatomic Ions
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an –ide ending, since the suffix is already present in the name of the anion. Examples are shown in Table 2. See Table 1 in Chapter 4.2 Ionic and Molecular Compounds for the list of common polyatomic ions.
| KC2H3O2, potassium acetate | (NH4)Cl, ammonium chloride | |
| NaHCO3, sodium bicarbonate | CaSO4, calcium sulfate | |
| Al2(CO3)3, aluminum carbonate | Mg3(PO4)2, magnesium phosphate | |
| Table 2. Names of Some Polyatomic Ionic Compounds | ||
Example 1
Name the following ionic compounds:
a) NaCl b) AlBr3 c) BaH2
Solution
a) Identify the cation and anion.
Na is a Group 1 metal, and thus it forms the cation Na+, called “sodium” ion.
Cl is a nonmetal, and forms the anion Cl–, chloride. Thus, NaCl = sodium chloride.
b) AlBr3 consists of aluminum and bromine; we call it aluminum bromide.
c) BaH2is called barium hydride.
Test Yourself
Name the following ionic compounds:
a) Al2S3 b) ZnS c) MgI2
Answers
a) aluminum sulfide b) zinc sulfide c) magnesium iodide
Compounds Containing a Metal Ion with a Variable Charge
Most of the transition metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ (see Figure 2 in Chapter 4.2 Ionic and Molecular Compounds), and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, “iron chloride,” will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Other examples are provided in Table 4.
| Transition Metal Ionic Compound | Name |
|---|---|
| FeCl3 | iron(III) chloride |
| Hg2O | mercury(I) oxide |
| HgO | mercury(II) oxide |
| Cu3(PO4)2 | copper(II) phosphate |
| Table 4. Names of Some Transition Metal Ionic Compounds | |
An old naming convention used the suffixes –ic and –ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl3, can be called called ferric chloride, and iron(II) chloride, FeCl2, is also known as ferrous chloride. This older naming convention remains in use by some segments of industry. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula SnF2, which is also named tin(II) fluoride following the more current convention. The other fluoride of tin is SnF4, is now named tin(IV) fluoride but is still often referred to as stannic fluoride. Knowing both convention remains important.
| Element | Common Ions | Common Names for Ions |
|---|---|---|
| Cu | Cu+/Cu2+ | cuprous/cupric |
| Fe | Fe2+/Fe3+ | ferrous/ferric |
| Co | Co2+/Co3+ | cobaltous/cobaltic |
| Cr | Cr2+/Cr3+ | chromous/chromic |
| Sn | Sn2+/Sn4+ | stannous/stannic |
| Pb | Pb2+/Pb4+ | plumbous/plumbic |
| Hg | Hg22+/Hg2+ | mercurous/mercuric |
| Table 5. Common Names for Some Metal Ions with Variable Charges | ||
Example 2
Name each species.
a) O2− b) Co c) Co2+
Solution
a) This species has a 2− charge on it, so it is an anion. Anions are named using the stem of the element name with the suffix -ide added. This is the oxide anion.
b) Because this species has no charge, it is an atom in its elemental form. This is cobalt.
c) In this case, there is a 2+ charge on the atom, so it is a cation. We note from Figure 2 in Chapter 4.2 Ionic and Molecular Compounds), that cobalt cations can have two possible charges, so the name of the ion must specify which charge the ion has. This is the cobalt(II) cation.
Test Yourself
Name each species: P3− and Sr2+
Answers
the phosphide anion and the strontium cation
Example 3
Name the following ionic compounds:
a) SnBr4 b) CoCl3 c) Fe2O3
Solution
a) First, identify the charge on the cation (Sn).
Because Br has a charge of –1, we know that Sn must have a charge of +4.
0 = 1(x) + 4(-1) x = +4 Therefore SnBr4= tin(IV) bromide
b) Cl adopts a charge of –1
0 = 1(x) + 3(-1) x = +3 Therefore CoCl3= cobalt(III) chloride
c) O adopts a charge of –2
0 = 2(x) + 3(-2) x = +3 Therefore Fe2O3= iron(III) oxide
Test Yourself
Name the following ionic compounds:
a) HgO b) PbCl4 c) PbS d) Sc2O3 e) AgCl
Answers
a) mercury(II) oxide b) lead(IV) chloride c) lead(II) sulphide
d) scandium oxide e) silver chloride
Example 4
Name the following ionic compounds:
a) Fe2S3 b) CuSe c) GaN d) CrCl3 e) Ti2(SO4)3 f) Co2O3 g) CaCl2 h) AlF3
Solution
The anions in these compounds have a fixed negative charge (S2−, Se2− , N3−, Cl−, SO42−, O−2, and F−), and the compounds must be neutral. Because the metal ions in questions a) to f) have a variable charge, we must figure out the charge of the metal ion by ensuring that the total number of positive charges in each compound must equal the total number of negative charges. Therefore the positive ions must be Fe3+, Cu2+, Ga3+, Cr3+, Ti3+ and Co3+. These charges are used in the names of the metal ions:
a) iron(III) sulfide b) copper(II) selenide c) gallium(III) nitride
d) chromium(III) chloride e) titanium(III) sulfate f) cobalt(III) oxide
In questions g) and h) the metal ions do not have a variable charge, therefore
g) Using the names of the ions, this ionic compound is named calcium chloride. It is not calcium(II) chloride because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+.
h)The name of this ionic compound is aluminum fluoride.
Test Yourself
Write the formulas of the following ionic compounds:
a) chromium(III) phosphide b) mercury(II) sulfide c) manganese(II) phosphate
d) copper(I) oxide e) chromium(VI) fluoride
Answers
a) CrP b) HgS c) Mn3(PO4)2 d) Cu2O e) CrF6
Erin Brockovich and Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich (Figure 1) discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr(VI) used by Pacific Gas & Electric (PG&E) to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich (for which Julia Roberts won an Oscar), Erin and lawyer Edward Masry sued PG&E for contaminating the water near Hinckley in 1993. The settlement they won in 1996—$333 million—was the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr(III) or Cr(VI) forms. Cr(III), an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr(VI) is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr(VI) can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Despite cleanup efforts, Cr(VI) groundwater contamination remains a problem in Hinckley and other locations across the globe. A 2010 study by the Environmental Working Group found that of 35 US cities tested, 31 had higher levels of Cr(VI) in their tap water than the public health goal of 0.02 parts per billion set by the California Environmental Protection Agency.
Example 5
Write the proper formula and give the proper name for each ionic compound formed between the two listed ions.
a) NH4+ and S2− b) Al3+ and PO43− c) Fe2+ and PO43−
Solution
a) Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. Enclosing the formula for the ammonium ion in parentheses, we have (NH4)2S. The compound’s name is ammonium sulfide.
b) Because the ions have the same magnitude of charge, we need only one of each to balance the charges. The formula is AlPO4, and the name of the compound is aluminum phosphate.
c) Neither charge is an exact multiple of the other, so we have to go to the least common multiple of 6. To get 6+, we need three iron(II) ions, and to get 6−, we need two phosphate ions. The proper formula is Fe3(PO4)2, and the compound’s name is iron(II) phosphate.
Test Yourself
Write the proper formula and give the proper name for each ionic compound formed between the two listed ions.
a) NH4+ and PO43− b) Co3+ and NO2−
Answers
a) (NH4)3PO4, ammonium phosphate b) Co(NO2)3, cobalt(III) nitrite
Ionic Compounds in Your Cabinets
Every day you encounter and use a large number of ionic compounds. Some of these compounds, where they are found, and what they are used for are listed in Table 3. Look at the label or ingredients list on the various products that you use during the next few days, and see if you run into any of those in this table, or find other ionic compounds that you could now name or write as a formula.
| Ionic Compound | Use |
|---|---|
| NaCl, sodium chloride | ordinary table salt |
| KI, potassium iodide | added to “iodized” salt for thyroid health |
| NaF, sodium fluoride | ingredient in toothpaste |
| NaHCO3, sodium bicarbonate | baking soda; used in cooking (and as antacid) |
| Na2CO3, sodium carbonate | washing soda; used in cleaning agents |
| NaOCl, sodium hypochlorite | active ingredient in household bleach |
| CaCO3 calcium carbonate | ingredient in antacids |
| Mg(OH)2, magnesium hydroxide | ingredient in antacids |
| Al(OH)3, aluminum hydroxide | ingredient in antacids |
| NaOH, sodium hydroxide | lye; used as drain cleaner |
| K3PO4, potassium phosphate | food additive (many purposes) |
| MgSO4, magnesium sulfate | added to purified water |
| Na2HPO4, sodium hydrogen phosphate | anti-caking agent; used in powdered products |
| Na2SO3, sodium sulfite | preservative |
| Table 3. Everyday Ionic Compounds | |
Nomenclature of Molecular (Covalent) Compounds
The bonding characteristics of inorganic molecular compounds are different from ionic compounds, and they are named using a different system as well. The charges of cations and anions dictate their ratios in ionic compounds, so specifying the names of the ions provides sufficient information to determine chemical formulas. However, because covalent bonding allows for significant variation in the combination ratios of the atoms in a molecule, the names for molecular compounds must explicitly identify these ratios.
Compounds Composed of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO2. Since these are different substances with different properties, they cannot both have the same name (they cannot both be called carbon oxide). To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element (the one farther to the left and/or bottom of the periodic table) is first, followed by the name of the more nonmetallic element (the one farther to the right and/or top) with its ending changed to the suffix –ide. The numbers of atoms of each element are designated by the Greek prefixes shown in Table 6.
| Number | Prefix | Number | Prefix |
|---|---|---|---|
| 1 (sometimes omitted) | mono- | 6 | hexa- |
| 2 | di- | 7 | hepta- |
| 3 | tri- | 8 | octa- |
| 4 | tetra- | 9 | nona- |
| 5 | penta- | 10 | deca- |
| Table 6. Nomenclature Prefixes | |||
When only one atom of the first element is present, the prefix mono– is usually deleted from that part. Thus, CO is named carbon monoxide, and CO2 is called carbon dioxide. When two vowels are adjacent, the a in the Greek prefix is usually dropped. Some other examples are shown in Table 7.
| Compound | Name | Compound | Name |
|---|---|---|---|
| SO2 | sulfur dioxide | BCl3 | boron trichloride |
| SO3 | sulfur trioxide | SF6 | sulfur hexafluoride |
| NO2 | nitrogen dioxide | PF5 | phosphorus pentafluoride |
| N2O4 | dinitrogen tetroxide | P4O10 | tetraphosphorus decaoxide |
| N2O5 | dinitrogen pentoxide | IF7 | iodine heptafluoride |
| Table 7. Names of Some Molecular Compounds Composed of Two Elements | |||
There are a few common names that you will encounter as you continue your study of chemistry. For example, although NO is often called nitric oxide, its proper name is nitrogen monoxide. Similarly, N2O is known as nitrous oxide even though our rules would specify the name dinitrogen monoxide. (And H2O is usually called water, not dihydrogen monoxide.) You should commit to memory the common names of compounds as you encounter them.
Example 6
Name the following covalent compounds:
a) SF6 b) N2O3 c) Cl2O7 d) P4O6 e) PF3 f) CO g) Se2Br2
Solution
Because these compounds consist solely of nonmetals, they are molecular compounds, therefore according to the rules, we use prefixes to designate the number of atoms of each element:
a) sulfur hexafluoride b) dinitrogen trioxide c) dichloride heptoxide
d) tetraphosphorus hexoxide e) phosphorus trifluoride f) carbon monoxide (not carbon monooxide)
g) diselenium dibromide
Test Yourself
Write the formulas for the following compounds:
a) phosphorus pentachloride b) dinitrogen monoxide c) iodine heptafluoride
d) carbon tetrachloride e) disulfur difluoride f) iodine pentabromide
Answers
a) PCl5 b) N2O c) IF7 d) CCl4 e) S2F2 f) IBr5

The following website provides practice with naming chemical compounds and writing chemical formulas. You can choose binary, polyatomic, and variable charge ionic compounds, as well as molecular compounds.
Binary Acids
Some compounds containing hydrogen are members of an important class of substances known as acids, and these compounds have interesting chemical properties. The chemistry of these compounds is explored in more detail in later chapters of this text, but for now, it will suffice to note that many acids release hydrogen ions, H+, when dissolved in water. To indicate that something is dissolved in water, we will use the phase label (aq) next to a chemical formula (where aq stands for “aqueous,” a word that describes something dissolved in water). To denote this distinct chemical property, a mixture of water with an acid is given a name derived from the compound’s name. If the compound is a binary acid (comprised of hydrogen and one other nonmetallic element):
- The word “hydrogen” is changed to the prefix hydro-
- The other nonmetallic element name is modified by adding the suffix –ic
- The word “acid” is added as a second word
For example, when the gas HCl (hydrogen chloride) is dissolved in water, the solution is called hydrochloric acid. Several other examples of this nomenclature are shown in Table 8.
| Name of Gas | Name of Acid |
|---|---|
| HF(g), hydrogen fluoride | HF(aq), hydrofluoric acid |
| HCl(g), hydrogen chloride | HCl(aq), hydrochloric acid |
| HBr(g), hydrogen bromide | HBr(aq), hydrobromic acid |
| HI(g), hydrogen iodide | HI(aq), hydroiodic acid |
| H2S(g), hydrogen sulfide | H2S(aq), hydrosulfuric acid |
| HCN(g), hydrogen cyanide | HCN(aq), hydrocyanic acid |
| Table 8. Names of Some Simple Acids | |
Oxyacids
Many compounds containing three or more elements (such as organic compounds or coordination compounds) are subject to specialized nomenclature rules that you will learn later. However, we will briefly discuss the important compounds known as oxyacids, compounds that contain hydrogen, oxygen, and at least one other element, and are bonded in such a way as to impart acidic properties to the compound (you will learn the details of this in a later chapter). Typical oxyacids consist of hydrogen combined with a polyatomic, oxygen-containing ion. To name oxyacids:
- Omit “hydrogen”
- Start with the root name of the anion
- Replace –ate with –ic, or –ite with –ous
- Add “acid”
For example, consider H2CO3 (which you might be tempted to call “hydrogen carbonate”). To name this correctly, “hydrogen” is omitted; the –ate of carbonate is replace with –ic; and acid is added—so its name is carbonic acid. Other examples are given in Table 9. There are some exceptions to the general naming method (e.g., H2SO4 is called sulfuric acid, not sulfic acid, and H2SO3 is sulfurous, not sulfous, acid).
| Formula | Anion Name | Acid Name |
|---|---|---|
| HC2H3O2 | acetate | acetic acid |
| HNO3 | nitrate | nitric acid |
| HNO2 | nitrite | nitrous acid |
| HClO4 | perchlorate | perchloric acid |
| HClO3 | chlorate | chloric acid |
| HClO2 | chlorite | chlorous acid |
| HClO | hypochlorite | hypochlorous acid |
| H2CO3 | carbonate | carbonic acid |
| H2SO4 | sulfate | sulfuric acid |
| H2SO3 | sulfite | sulfurous acid |
| H3PO4 | phosphate | phosphoric acid |
| H3PO3 | phosphite | phosphorous acid |
| H2CrO4 | chromate | chromic acid |
| Table 9. Names of Common Oxyacids | ||
Example 7
Name each acid without consulting the tables.
a) HBr(aq) b) H2SO4 c) HF(g) d) HCN(aq) e) H2S(aq)
Solution
a) As an aqueous binary acid, the acid’s name is hydro- + stem name + -ic acid. Because this acid contains a bromine atom, the name is hydrobromic acid.
b) Because this acid is derived from the sulfate ion, the name of the acid is the stem of the anion name + -ic acid. The name of this acid is sulfuric acid.
c) Because HF(g) is in gaseous form, we name it hydrogen fluoride.
d) HCN(aq) contains the polyatomic ion cyanide. The root is “cyan”, thus HCN(aq) = hydrocyanic acid.
e) H2S(aq) contains the ion sulfide. In this case, however, the root takes a slightly different form of “sulfur” (the same as the element name). Thus H2S(aq) = hydrosulfuric acid.
Test Yourself
Name each acid.
a) HF(aq) b) HNO2 c) HClO4 d) H2SO4 e) H2CrO4(aq) f) H3PO4(aq) g) HClO(aq)
Answers
a) hydrofluoric acid b) nitrous acid c) perchloric acid d) sulphuric acid e) chromic acid
f) phosphoric acid g) hypochlorous acid
All acids have some similar properties. For example, acids have a sour taste; in fact, the sour taste of some of our foods, such as citrus fruits and vinegar, is caused by the presence of acids in food. Many acids react with some metallic elements to form metal ions and elemental hydrogen. Acids make certain plant pigments change colors; indeed, the ripening of some fruits and vegetables is caused by the formation or destruction of excess acid in the plant. In a later chapter, we will explore the chemical behaviour of acids.
Acids are very prevalent in the world around us. We have already mentioned that citrus fruits contain acid; among other compounds, they contain citric acid, H3C6H5O7(aq). Oxalic acid, H2C2O4(aq), is found in spinach and other green leafy vegetables. Hydrochloric acid not only is found in the stomach (stomach acid) but also can be bought in hardware stores as a cleaner for concrete and masonry. Phosphoric acid is an ingredient in some soft drinks.
Sodium in Your Food
The element sodium, at least in its ionic form as Na+, is a necessary nutrient for humans to live. In fact, the human body is approximately 0.15% sodium, with the average person having one-twentieth to one-tenth of a kilogram in their body at any given time, mostly in fluids outside cells and in other bodily fluids.
Sodium is also present in our diet. The common table salt we use on our foods is an ionic sodium compound. Many processed foods also contain significant amounts of sodium added to them as a variety of ionic compounds. Why are sodium compounds used so much? Usually sodium compounds are inexpensive, but, more importantly, most ionic sodium compounds dissolve easily. This allows processed food manufacturers to add sodium-containing substances to food mixtures and know that the compound will dissolve and distribute evenly throughout the food. Simple ionic compounds such as sodium nitrite (NaNO2) are added to cured meats, such as bacon and deli-style meats, while a compound called sodium benzoate is added to many packaged foods as a preservative. Table 10 “Some Sodium Compounds Added to Food” is a partial list of some sodium additives used in food. Some of them you may recognize after reading this chapter. Others you may not recognize, but they are all ionic sodium compounds with some negatively charged ion also present.
| Sodium Compound | Use in Food |
|---|---|
| Sodium acetate | preservative, acidity regulator |
| Sodium adipate | food acid |
| Sodium alginate | thickener, vegetable gum, stabilizer, gelling agent, emulsifier |
| Sodium aluminum phosphate | acidity regulator, emulsifier |
| Sodium aluminosilicate | anticaking agent |
| Sodium ascorbate | antioxidant |
| Sodium benzoate | preservative |
| Sodium bicarbonate | mineral salt |
| Sodium bisulfite | preservative, antioxidant |
| Sodium carbonate | mineral salt |
| Sodium carboxymethylcellulose | emulsifier |
| Sodium citrates | food acid |
| Sodium dehydroacetate | preservative |
| Sodium erythorbate | antioxidant |
| Sodium erythorbin | antioxidant |
| Sodium ethyl para-hydroxybenzoate | preservative |
| Sodium ferrocyanide | anticaking agent |
| Sodium formate | preservative |
| Sodium fumarate | food acid |
| Sodium gluconate | stabilizer |
| Sodium hydrogen acetate | preservative, acidity regulator |
| Sodium hydroxide | mineral salt |
| Sodium lactate | food acid |
| Sodium malate | food acid |
| Sodium metabisulfite | preservative, antioxidant, bleaching agent |
| Sodium methyl para-hydroxybenzoate | preservative |
| Sodium nitrate | preservative, color fixative |
| Sodium nitrite | preservative, color fixative |
| Sodium orthophenyl phenol | preservative |
| Sodium propionate | preservative |
| Sodium propyl para-hydroxybenzoate | preservative |
| Sodium sorbate | preservative |
| Sodium stearoyl lactylate | emulsifier |
| Sodium succinates | acidity regulator, flavour enhancer |
| Sodium salts of fatty acids | emulsifier, stabilizer, anticaking agent |
| Sodium sulfite | mineral salt, preservative, antioxidant |
| Sodium sulfite | preservative, antioxidant |
| Sodium tartrate | food acid |
| Sodium tetraborate | preservative |
Table 10. Some Sodium Compounds Added to Food
The use of so many sodium compounds in prepared and processed foods has alarmed some physicians and nutritionists. They argue that the average person consumes too much sodium from his or her diet. The average person needs only about 500 mg of sodium every day; most people consume more than this—up to 10 times as much. Some studies have implicated increased sodium intake with high blood pressure; newer studies suggest that the link is questionable. However, there has been a push to reduce the amount of sodium most people ingest every day: avoid processed and manufactured foods, read labels on packaged foods (which include an indication of the sodium content), don’t oversalt foods, and use other herbs and spices besides salt in cooking.
Key Concepts and Summary
Chemists use nomenclature rules to clearly name compounds. Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to –ide. For example, K2O is called potassium oxide. If the metal can form ions with different charges, a Roman numeral in parentheses follows the name of the metal to specify its charge. Thus, FeCl2 is iron(II) chloride and FeCl3 is iron(III) chloride. Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. Molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. Binary acids are named using the prefix hydro-, changing the –ide suffix to –ic, and adding “acid;” HCl is hydrochloric acid. Oxyacids are named by changing the ending of the anion to –ic, and adding “acid;” H2CO3 is carbonic acid.
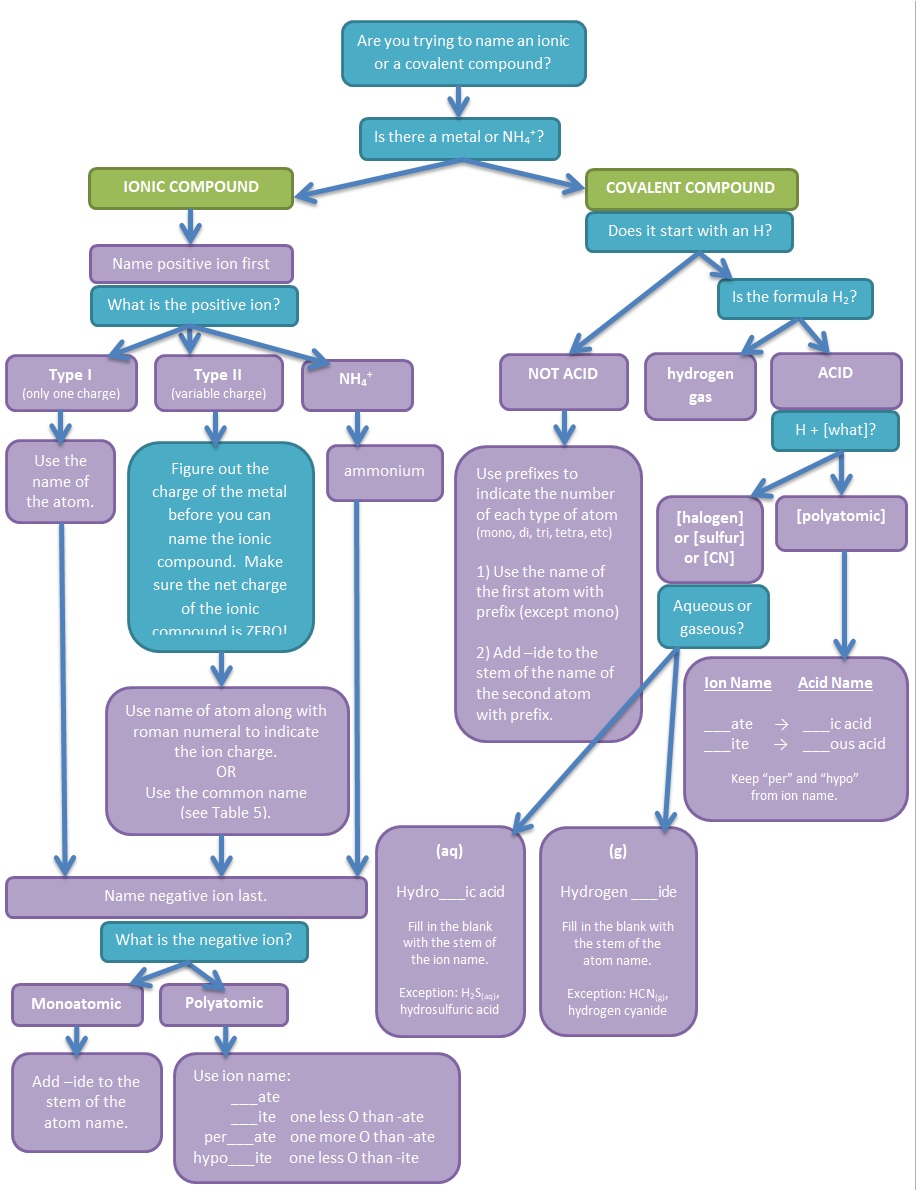
Figure 3. Flowchart illustrating the thought process involved in naming simple ionic and covalent compounds and the rules needed to follow.
Exercises
1. Give the formula and name for each ionic compound formed between the two listed ions.
a) Mg2+ and Cl− b) Fe2+ and O2− c) Fe3+ and O2−
2. Give the formula and name for each ionic compound formed between the two listed ions.
a) Cu2+ and F− b) Ca2+ and O2− c) K+ and P3−
3. Give the formula and name for each ionic compound formed between the two listed ions.
a) K+ and SO42− b) NH4+ and S2− c) NH4+ and PO43−
4. Give the formula and name for each ionic compound formed between the two listed ions.
a) Pb4+ and SO42− b) Na+ and I3− c) Li+ and Cr2O72−
5. Give the formula and name for each ionic compound formed between the two listed ions.
a) Ag+ and SO32− b) Na+ and HCO3− c) Fe3+ and ClO3−
6. .Which of these formulas represent molecules? State how many atoms are in each molecule.
a) Fe b) PCl3 c) P4 d) Ar
7. What is the difference between CO and Co?
8. Give the proper formula for each diatomic element.
9. What is the stem of fluorine used in molecule names? CF4 is one example.
10. Give the proper name for each molecule.
a) PF3 b) TeCl2 c) N2O3
11. Give the proper name for each molecule.
a) XeF2 b) O2F2 c) SF6
12. Give the proper name for each molecule.
a) N2O b) N2O4 c) N2O5
13. Give the proper formula for each name.
a) dinitrogen pentoxide b) tetraboron tricarbide c) phosphorus pentachloride
14. Give the proper formula for each name.
a) dioxygen dichloride b) dinitrogen trisulfide c) xenon tetrafluoride
15. Give the proper formula for each name.
a) iodine trifluoride b) xenon trioxide c) disulfur decafluoride
16. Give the formula for each acid.
a) perchloric acid b) hydroiodic acid
17. Name each acid.
a) HF(aq) b) HNO3(aq) c) H2C2O4(aq)
18. Name the following compounds:
a) CsCl b) BaO c) K2S d) BeCl2 e) HBr f) AlF3
19. Write the formulas of the following compounds:
a) rubidium bromide b) magnesium selenide c) sodium oxide d) calcium chloride
e) hydrogen fluoride f) gallium phosphide g) aluminum bromide h) ammonium sulfate
20. Write the formulas of the following compounds:
a) chlorine dioxide b) dinitrogen tetraoxide c) potassium phosphide
d) silver(I) sulfide e) aluminum nitride f) silicon dioxide
21. Each of the following compounds contains a metal that can exhibit more than one ionic charge. Name these compounds:
a) Cr2O3 b) FeCl2 c) CrO3 d) TiCl4 e) CoO f) MoS2
22. The following ionic compounds are found in common household products. Write the formulas for each compound:
a) potassium phosphate b) copper(II) sulfate c) calcium chloride
d) titanium dioxide e) ammonium nitrate
f) sodium bisulfate (the common name for sodium hydrogen sulfate)
23. What are the IUPAC names of the following compounds?
a) manganese dioxide b) mercurous chloride (Hg2Cl2) c) ferric nitrate [Fe(NO3)3]
d) titanium tetrachloride e) cupric bromide (CuBr2)
Answers
1. a) magnesium chloride, MgCl2 b) iron(II) oxide, FeO c) iron(III) oxide, Fe2O3
2. a) copper(II) fluoride, CuF2 b) calcium oxide, CaO c) potassium phosphide, K3P
3. a) potassium sulfate, K2SO4 b) ammonium sulfide, (NH4)2S c) ammonium phosphate, (NH4)3PO4
4. a) lead(IV) sulfate, Pb(SO4)2 b) sodium triiodide, NaI3 c) lithium dichromate, Li2Cr2O7
5. a) silver sulfite, Ag2SO3 b) sodium hydrogen carbonate, NaHCO3 c) iron(III) chlorate, Fe(ClO3)3
6. a) not a molecule b) a molecule; four atoms total c) a molecule; four atoms total
d) not a molecule
7. CO is a compound of carbon and oxygen; Co is the element cobalt.
8. H2, O2, N2, F2, Cl2, Br2, I2
9. fluor-
10. a) phosphorus trifluoride b) tellurium dichloride c) dinitrogen trioxide
11. a) xenon difluoride b) dioxygen difluoride c) sulfur hexafluoride
12. a) dinitrogen monoxide b) dinitrogen tetroxide c) dinitrogen pentoxide
13. a) N2O5 b) B4C3 c) PCl5
14. a) O2Cl2 b) N2S3 c) XeF4
15. a) IF3 b) XeO3 c) S2F10
16. a) HClO4(aq) b) HI(aq)
17. a) hydrofluoric acid b) nitric acid c) oxalic acid
18. a) cesium chloride b) barium oxide c) potassium sulfide
d) beryllium chloride e) hydrogen bromide f) aluminum fluoride
19. a) RbBr b) MgSe c) Na2O d) CaCl2 e) HF f) GaP g) AlBr3 h) (NH4)2SO4
20. a) ClO2 b) N2O4 c) K3P d) Ag2S e) AlN f) SiO2
21. a) chromium(III) oxide b) iron(II) chloride c) chromium(VI) oxide
d) titanium(IV) chloride e) cobalt(II) oxide f) molybdenum(IV) sulfide
22. a) K3PO4 b) CuSO4 c) CaCl2 d) TiO2 e) NH4NO3 f) NaHSO4
23. a) manganese(IV) oxide b) mercury(I) chloride c) iron(III) nitrate
d) titanium(IV) chloride e) copper(II) bromide
Glossary
binary acid: compound that contains hydrogen and one other element, bonded in a way that imparts acidic properties to the compound (ability to release H+ ions when dissolved in water)
binary compound: compound containing two different elements.
nomenclature: system of rules for naming objects of interest
oxyacid: compound that contains hydrogen, oxygen, and one other element, bonded in a way that imparts acidic properties to the compound (ability to release H+ ions when dissolved in water)