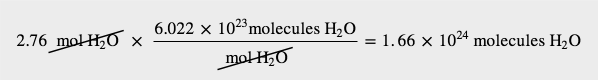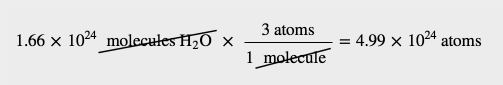Learning Objectives
By the end of this section, you will be able to:
- Define the mole and describe why it is useful
- Use mass and mole concepts to solve factor-label problems
From Weighing to Counting
Atomic and molecular mass provides a way to understand/predict the weights of substances in a reaction. For example, consider:
C(s) + O2(g) → CO2(g)
This equation described how carbon reacts with oxygen gas to produce carbon dioxide in a 1:1:1 ratio. In other words, the equation tells us that 1 atom of carbon reacts with 1 molecule of O2 to form 1 molecule of CO2, or 500 C atoms will react with 500 O2 molecules to form 500 molecules for CO2, and so on. It suggests we need an equal number of C atoms and O2 molecules for the reactants to completely convert to product.
It is impractical to actually count out a number of atoms or molecules. But we can approximate this counting by weighing carefully and utilizing the formula mass.
Example 1
How many grams should you weigh to get 5000 bolts if each bolt has an average mass of 0.25g?
Solution
[latex]5000\;\rule[0.75ex]{2.0em}{0.1ex}\hspace{-2.0em}\text{bolts} \times \frac{0.25\;\text{g}}{1\;\rule[0.25ex]{1.25em}{0.1ex}\hspace{-1.25em}\text{bolt}} = 1.2\times 10^3\;\text{g are needed}[/latex]
Test Yourself
If bolts have an average mass of 0.25g, how many bolts are present in 62.5g of bolts?
Answer
2.5×102 bolts
Furthermore, if two different types of things are measured by weighing, the same number of each will be present if the ratio of the masses weighed out is equal to the ratio of masses of the individual units.
For example: If 1 bolt weighs 0.25 g and 1 nut weighs 0.50 g, then 300 g of bolts will contain the same number of items as 600 g of nuts.
Why? 1 nut weighs twice as much as 1 bolt, therefore you must have the same number of each item when you weigh out a total mass of nuts that’s twice the total mass of bolts.
Proof:
[latex]300\;\rule[0.75ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g bolts} \times \frac{1\;\text{bolt}}{0.25\;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g bolts}} = 1200\;\text{bolts}[/latex]
[latex]600\;\rule[0.75ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g nuts} \times \frac{1\;\text{nut}}{0.50\;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g nuts}} = 1200\;\text{nuts}[/latex]
It is the same idea, with:
2 kg nuts & 1 kg bolts, or
2 tons nuts & 1 ton bolts, or
2 dozen tons of nuts & 1 dozen tons of bolts…
We know that in every case the number of nuts is the same as the number of bolts.
Note: in each example, we don’t necessarily know what the number of items is, but we can be sure that it’s the same number of each item.
The same logic applies to chemical substances.
For the above reaction, C(s) + O2(g) → CO2(g), as long as we weigh out amounts of C and O2 in the same ratio as the weights of 1 C atom (12.011 amu) and 1 O2 molecule (31.9988 amu), we can be sure we’ll have an equal number of C atoms and O2 molecules.
Now, the easiest way to choose total weights in the desired ratio—without doing any math—is to just mimic the numerical values of the known atomic and molecular weights. For example, to make sure we have a 12.011 to 31.9988 weight ratio of C and O2, we could just weigh out:
12.011 grams of C and 31.9988 grams of O2
or 12.011 kg of C and 31.9988 kg of O2
or 12.011 lbs of C and 31.9988 lbs of O2
or 12.011 tons of C and 31.9988 tons of O2
or etc.. They all will have equal numbers of C atoms and O2 molecules.
The Mole
The identity of a substance is defined not only by the types of atoms or ions it contains, but by the quantity of each type of atom or ion. For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, because a hydrogen peroxide molecule contains two oxygen atoms, as opposed to the water molecule, which has only one, the two substances exhibit very different properties. Today, we possess sophisticated instruments that allow the direct measurement of these defining microscopic traits; however, the same traits were originally derived from the measurement of macroscopic properties (the masses and volumes of bulk quantities of matter) using relatively simple tools (balances and volumetric glassware). This experimental approach required the introduction of a unit for amount of substances, the mole, which remains indispensable in modern chemical science.
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a macroscopic, bulk sample of matter.
Estimating the numerical value of Avogadro’s number
The mole, the SI unit for substance, is a unit for counting, and it’s often said that we can think of the mole as like a dozen. A subtle difference, however is that a dozen is defined as a specific number (12) of items, but a mole (Avogadro’s number of items) is defined as whatever number of H atoms you’d have if you weighed out 1.00794 g of H, (or S atoms in 32.066 g of S, or 12C atoms in 12 g of 12C, and so on).
This usually isn’t a problem, as these terms are normally encountered. If we bought a dozen donuts, we’d want to know how many we got—is there one for each person, etc.. But if we weigh out a mole of Na (22.9898 g) and a mole of Cl (35.4527 g), we probably don’t care how many atoms we have—we’re just happy to know we have the same number of atoms of each, in order to make NaCl without any atoms of Na or Cl leftover.
However, in some relatively rare circumstances, we may need to know how many atoms or molecules do we have when we weigh out a certain amount. For this, we’d actually like to know a numerical value for Avogadro’s number. To get this value, someone has to measure how many H atoms are there in 1.00794 g of H, (or S atoms in 32.066 g of S, or 12C atoms in 12 g of 12C, or…). To do that, someone has to measure the mass, in grams, of a single atom.
Many different ways have been dreamed up for estimating, in effect, the mass of a single atom. One very good one is to weigh, in grams, a crystal of a measured volume, and use the diffraction of x-rays to measure the distance between adjacent atoms, and thus estimate the number of atoms in the crystal. Calculating the number of things in a mole (Avogadro’s number) can be illustrated using 12C as an example. 12C provides a good basis because it’s the only substance where we can start with the mass of an atom in amu exactly (12 g).
First, from the definition of a mole: one 12C atom weighs 12 amu’s (exactly),
so a mole of 12C means 12 g of 12C (exactly).
The current best measurements give the mass of a 12C atom to 8 significant figures as 1.9926465 x 10-23g, so to determine Avogadro’s number we ask how many times 1.99264654 x 10-23g goes into 12 g (exactly).
This works out as:
[latex]latex \frac{12\;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g (exactly) /mole}}{1.99264654\times 10^{-23}\;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g/atom}} = 6.0221418\times 10^{23}\;\text{atoms/mole}[/latex]
So, based on current best measurements, we can write:
1 Mole of things = Avogadro’s number of them
Avogadro’s number = 6.0221418 x 1023 = 6.022 x 1023 approximately
Remember: this value, 6.022… x 1023, refers to entities/particles/atoms/ molecules/ions —whatever you have a mole of—per mole, just like dozen can be for muffins, donuts, cookies, etc….
But notice! The value of this number is not used as frequently as some students would assume. What is valuable to scientists on a day-to-day basis is the idea of the mole, that the ratio of the masses of substances is unchanging at any scale.
Therefore, a mole is defined as the amount of substance containing the same number of discrete entities (such as atoms, molecules, and ions) as the number of atoms in a sample of pure 12C weighing exactly 12 g.The number of entities composing a mole has been experimentally determined to be 6.0221418 × 1023, a fundamental constant named Avogadro’s number (NA) or the Avogadro constant in honor of Italian scientist Amedeo Avogadro.
But most importantly, the mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, ions, etc..
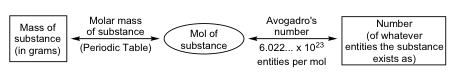
Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (see Figure 1).

Because the definitions of both the mole and the atomic mass unit are based on the same reference substance, 12C, the molar mass of any substance is numerically equivalent to its atomic or formula weight in amu. According to the definition of the mole, 12 g of 12C contains 1 mole of 12C atoms. Its molar mass is 12 g/mol.
This relationship holds for all elements. Extending this principle, the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in amu (Figure 2).

| Element | Average Atomic Mass (amu) | Molar Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.011 | 12.011 | 6.022 × 1023 |
| H | 1.00794 | 1.00794 | 6.022 × 1023 |
| O | 15.9994 | 15.9994 | 6.022 × 1023 |
| Na | 22.9898 | 22.9898 | 6.022 × 1023 |
| Cl | 35.4527 | 35.4527 | 6.022 × 1023 |
| Table 1. | |||
While atomic mass and molar mass have the same value (are numerically equal), they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units (amu versus g).
To appreciate the enormity of the mole, consider a small drop of water weighing about 0.03 g (see Figure 3). Although this represents just a tiny fraction of 1 mole of water (~18 g), it contains more water molecules than can be clearly imagined. If the molecules were distributed equally among the roughly seven billion people on earth, each person would receive more than 100 billion molecules.


The mole is used in chemistry to represent 6.022 × 1023 of something, but it can be difficult to conceptualize such a large number. Watch this video and then complete the “Think” questions that follow. Explore more about the mole by reviewing the information under “Dig Deeper.”
The relationships between formula mass, the mole, and Avogadro’s number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance’s mass.
Example 2
According to nutritional guidelines from the US Department of Agriculture, the estimated average requirement for dietary potassium is 4.7 g. What is the estimated average requirement of potassium in moles?
Solution
- The mass in grams of K is provided, and the question asks us to find K in moles.
- Referring to the periodic table, the atomic mass of K is 39.0983 amu, and so its molar mass is 39.0983 g/mol.
- The given mass of K (4.7 g) is a bit more than one-tenth the molar mass (39.0983 g), so a reasonable “ballpark” estimate of the number of moles would be slightly greater than 0.1 mol.
The molar amount of a substance may be calculated by dividing its mass (g) by its molar mass (g/mol):

The factor-label method supports this mathematical approach since the unit “g” cancels and the answer has units of “mol:”
The calculated magnitude (0.12 mol K) is consistent with our ballpark expectation, since it is a bit greater than 0.1 mol.
Test Yourself
Beryllium is a light metal used to fabricate transparent X-ray windows for medical imaging instruments. How many moles of Be are in a thin-foil window weighing 3.24 g?
Set up the conversion using the factor-label method, so that your solution not only gives an answer but provides an explanation for how you found it.
Answer
0.360mol
Example 3
A liter of air contains 9.2 × 10−4 mol argon. What is the mass of Ar in a liter of air?
Solution
- The molar amount of Ar is given. The problem asks us for mass (grams).
- The periodic table shows the mass of Ar is 39.9 amu, so Ar is 39.9 grams/mole.
- Since the amount of Ar is less than 1 mole, the mass will be less than the mass of 1 mole of Ar. The molar amount in question is approximately one-one thousandth (1 x 10−3) of a mole, and so the corresponding mass is expected to be roughly one-one thousandth of the molar mass (~0.04 g).

In this case, logic dictates (and the factor-label method supports) multiplying the provided amount (mol) by the molar mass (g/mol):
The result is in agreement with our expectations, around 0.04 g of argon.
Test Yourself
What is the mass of 2.561 mol of gold? Again, set up the problem with factor-label to show the process you undertake to arrive at the answer.
Answer
504.4 g
Example 4
Copper is commonly used to fabricate electrical wire (Figure 4). How many copper atoms are in 5.00 g of copper wire?

Solution
- Mass of copper (Cu) is given. The problem asks us to identify the number of atoms. We don’t have a direct conversion to this, though moles is essentially a count. If we get moles we can use Avogadro’s number (NA) to convert this molar amount to number of Cu atoms. We will need to make two conversions in sequence.
- The periodic table shows the mass of a copper atom is 63.55 amu, so copper is 63.55 grams/mole.
- Considering that the provided sample mass (5.00 g) is a little less than one-tenth the mass of 1 mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would be around one-tenth NA, or approximately 1022 Cu atoms.

The factor-label method yields the desired cancellation of units, and the computed result is on the order of 1022 as expected.
Test Yourself
A prospector panning for gold in a river collects 15.00 g of pure gold. How many Au atoms are in this quantity of gold? Use factor-label and show your reasoning to communicate the process you used to find the answer.
Answer
4.586 × 1022 Au atoms
We can calculate the total molar mass of a compound the same way we calculated total molecular mass—just replace amu (for molecular mass) with grams (for molar mass)—but keep in mind the difference between what these two terms mean.
Example 5
Our bodies synthesize protein from amino acids. One of these amino acids is glycine, which has the molecular formula C2H5O2N. How many moles of glycine molecules are contained in 28.35 g of glycine?
Solution
- We are given the mass of glycine, and are asked to find moles of glycine.
- The molar mass (grams/mole) of glycine is required for this calculation, and it is computed in the same fashion as its molecular mass.
- The provided mass of glycine (~28 g) is a bit more than one-third the molar mass (~75 g/mol), so we would expect the computed result to be a bit greater than one-third of a mole (~0.33 mol).
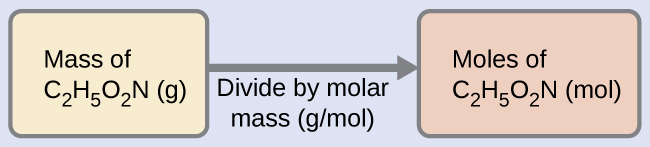
One mole of glycine, C2H5O2N, contains 2 moles of carbon, 5 moles of hydrogen, 2 moles of oxygen, and 1 mole of nitrogen:
| 2 C mass = 2 x 12.011 g/mol | = 24.022 g/mol |
| 5 H masses = 5 x 1.00794 g/mol | = 5.0397 g/mol |
| 2 O masses = 2 x 15.9994 g/mol | = 31.9988 g/mol |
| 1 N masses = 1 x 14.0067 g/mol | = 14.0067 g/mol |
| Total
|
= 75.067 g/mol = the molar mass of C2H5O2N |
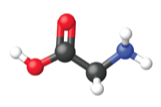
Figure 5. The average mass of a mole of glycine, C2H5O2N, is 75.067 g/mol, which is the sum of the average molar masses of each of its constituent atoms. The molecular structure of glycine.
Dividing the compound’s mass by its molar mass yields:
This result is consistent with our rough estimate.
Test Yourself
How many moles of sucrose, C12H22O11, are in a 25-g sample of sucrose?
Answer
0.073 mol
Example 6
Vitamin C is a covalent compound with the molecular formula C6H8O6. The recommended daily dietary allowance of vitamin C for children aged 4–8 years is 1.42 × 10−4 mol. What is the mass of this allowance in grams?
Solution
- We are given the number of moles of vitamin C, and asked to find its mass (grams).
- Consulting a periodic table with the formula in hand, we can calculate that vitamin C is 176.126 g/mol. We could also find this value by consulting a reputable source for the molar mass.
- The given number of moles is a very small fraction of a mole (~10−4 or one-ten thousandth); therefore, we would expect the corresponding mass to be about one-ten thousandth of the molar mass (~0.02 g).

Performing the calculation, we get:
This is consistent with the anticipated result.
Test Yourself
What is the mass of 0.443 mol of hydrazine, N2H4?
Answer
14.2 g
Counting Neurotransmitter Molecules in the Brain
The brain is the control center of the central nervous system (Figure 6). It sends and receives signals to and from muscles and other internal organs to monitor and control their functions; it processes stimuli detected by sensory organs to guide interactions with the external world; and it houses the complex physiological processes that give rise to our intellect and emotions. The broad field of neuroscience spans all aspects of the structure and function of the central nervous system, including research on the anatomy and physiology of the brain. Great progress has been made in brain research over the past few decades, and the BRAIN Initiative, a federal initiative announced in 2013, aims to accelerate and capitalize on these advances through the concerted efforts of various industrial, academic, and government agencies.
Specialized cells called neurons transmit information between different parts of the central nervous system by way of electrical and chemical signals. Chemical signaling occurs at the interface between different neurons when one of the cells releases molecules called neurotransmitters that diffuse across the small gap between the cells and bind to the surface of the other cell. These neurotransmitter molecules are stored in small intracellular structures called vesicles that fuse to the cell wall and then break open to release their contents when the neuron is appropriately stimulated. This process is called exocytosis (see Figure 7). One neurotransmitter that has been very extensively studied is dopamine, C8H11NO2. Dopamine is involved in various neurological processes that impact a wide variety of human behaviors. Dysfunctions in the dopamine systems of the brain underlie serious neurological diseases such as Parkinson’s and schizophrenia.
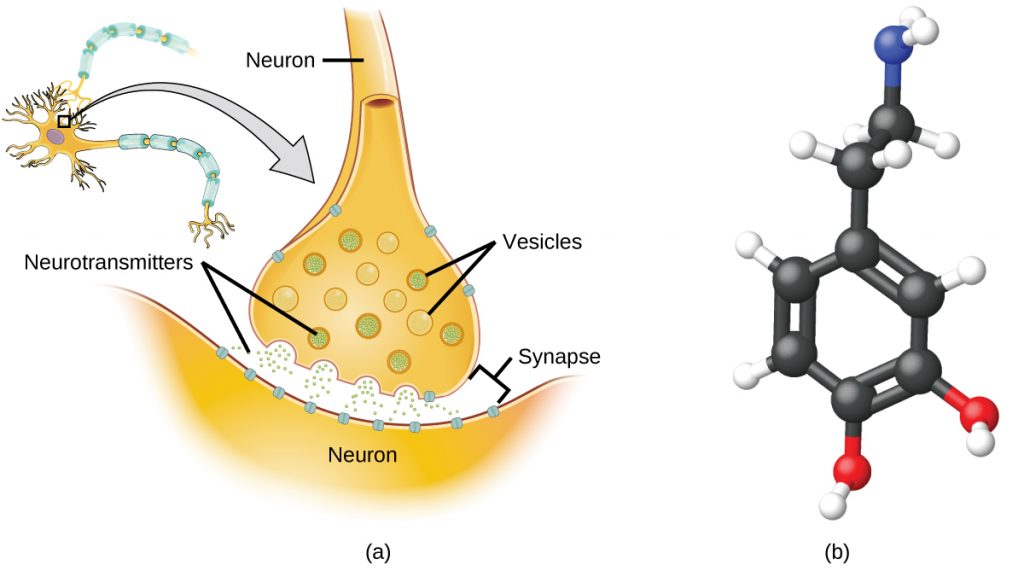
One important aspect of the complex processes related to dopamine signaling is the number of neurotransmitter molecules released during exocytosis. Since this number is a central factor in determining neurological response (and subsequent human thought and action), it is important to know how this number changes with certain controlled stimulations, such as the administration of drugs. It is also important to understand the mechanism responsible for any changes in the number of neurotransmitter molecules released—for example, some dysfunction in exocytosis, a change in the number of vesicles in the neuron, or a change in the number of neurotransmitter molecules in each vesicle.
Significant progress has been made recently in directly measuring the number of dopamine molecules stored in individual vesicles and the amount actually released when the vesicle undergoes exocytosis. Using miniaturized probes that can selectively detect dopamine molecules in very small amounts, scientists have determined that the vesicles of a certain type of mouse brain neuron contain an average of 30,000 dopamine molecules per vesicle (about 5 × 10−20 mol or 50 zmol). Analysis of these neurons from mice subjected to various drug therapies shows significant changes in the average number of dopamine molecules contained in individual vesicles, increasing or decreasing by up to three-fold, depending on the specific drug used. These studies also indicate that not all of the dopamine in a given vesicle is released during exocytosis, suggesting that it may be possible to regulate the fraction released using pharmaceutical therapies.[1]
Summary of using the mole idea:
Note:
- factor-label is both a general problem solving strategy and a way to communicate your process to others. Set up and solve calculations using it, including labels and paying attention to precision.
- learn the approach to solving problems, as it will translate to other types of problems. The approach in the Examples above is shown in the bullet-point lists for each example.
- metric and other additional conversions can be added to our solution “road maps”.
- there is often more than one correct route to a solution.
More Worked Out Problems
Example 7
How many molecules are present in 2.76 mol of H2O? How many atoms is this?
Solution
- What (number and label) is given and what (number and label) are we expected to find?
- What ratios are available to us that may help us make the conversion? Find any necessary atomic or formula masses, etc.
- Can you make a rough estimate of the answer?
The definition of a mole is an equality that can be used to construct a conversion factor. Also, because we know that there are three atoms in each molecule of H2O, we can also determine the number of atoms in the sample.
To determine the total number of atoms, we have
Test Yourself
How many molecules are present in 4.61 × 10−2 mol of O2?
Answer
2.78 × 1022 molecules
Example 8
What is the molar mass of C6H12O6?
Solution
To determine the molar mass, we simply add the atomic masses of the atoms in the molecular formula but express the total in grams per mole, not atomic mass units. The masses of the atoms can be taken from the periodic table:
| 6 C = 6 × 12.011 | = 72.066 |
| 12 H = 12 × 1.00794 | = 12.09528 |
| 6 O = 6 × 15.9994 | = 95.9964 |
| TOTAL | = 180.158 g/mol |
Per convention, the unit grams per mole is written as a fraction.
Test Yourself
What is the molar mass of AgNO3?
Answer
169.873 g/mol
Example 9
What is the mass of 3.56 mol of HgCl2? The molar mass of HgCl2 is 271.50 g/mol.
Solution
- What (number and label) is given and what (number and label) are we expected to find?
- What ratios are available to us that may help us make the conversion? Find any necessary atomic or formula masses, etc.
- Can you make a rough estimate of the answer?
Use the molar mass as a conversion factor between moles and grams. Because we want to cancel the mole unit and introduce the gram unit, we can use the molar mass as given:
[latex]3.56\; \rule[0.5ex]{1.25em}{0.1ex}\hspace{-1.25em}\text{mol}\; \text{HgCl}_2 \times \frac{271.50\; \text{g HgCl}_2} {1 \;\rule[0.25ex]{1.25em}{0.1ex}\hspace{-1.25em}\text{mol}\; \text{HgCl}_2}[/latex] [latex]= 967\; \text{g}\; \text{HgCl}_2[/latex]
Test Yourself
What is the mass of 33.7 mol of H2O?
Answer
607 g
Example 10
How many moles of H2O are present in 240.0 g of water (about the mass of a cup of water)?
Solution
Use the molar mass of H2O as a conversion factor from mass to moles. The molar mass of water is (1.00794 + 1.00794 + 15.9994) = 18.0153 g/mol. However, because we want to cancel the gram unit and introduce moles, we need to take the reciprocal of this quantity, or 1 mol/18.0153 g:
[latex]240.0\; \rule[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}\; \text{H}_2\text{O} \times \frac{1\; \text{mol H}_2\text{O}} {18.0153\; \rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}\; \text{H}_2\text{O}}[/latex] [latex]= 13.32\; \text{mol}\; \text{H}_2\text{O}[/latex]
Test Yourself
How many moles are present in 35.6 g of H2SO4 (molar mass = 98.079 g/mol)?
Answer
0.363 mol
Example 11
The density of ethanol is 0.789 g/mL. How many moles are in 100.0 mL of ethanol? The molar mass of ethanol is 46.069 g/mol.
Solution
Here, we use density to convert from volume to mass and then use the molar mass to determine the number of moles.
[latex]100.0\; \rule[0.5ex]{0.8em}{0.1ex}\hspace{-0.8em}\text{mL ethanol}\; \times \frac{0.789\; \text{g}} {1\; \rule[0.25ex]{0.8em}{0.1ex}\hspace{-0.8em}\text{mL}} \times \frac{1\; \text{mol}} {46.069\; \rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}}[/latex] [latex]= 1.71\; \text{mol ethanol}[/latex]
Test Yourself
If the density of benzene, C6H6, is 0.879 g/mL, how many moles are present in 17.9 mL of benzene?
Answer
0.201 mol
Key Concepts and Summary
A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. The mass in grams of 1 mole of substance is its molar mass. Chemists use these relationships as well as others to perform unit conversions, solving problems via an approach referred to as factor-label.
Review-Reflect, Extend
Review-Reflect
1. Which contains the greatest mass of oxygen: 0.75 mol of ethanol (C2H5OH), 0.60 mol of formic acid (HCO2H), or 1.0 mol of water (H2O)? Explain why.
2. How are the molecular mass and the molar mass of a compound similar and how are they different?
3. Calculate the molar mass of each of the following:
a) the anesthetic halothane, C2HBrClF3
b) the herbicide paraquat, C12H14N2Cl2
c) caffeine, C8H10N4O2
4. Determine the mass of each of the following:
a) 0.0146 mol KOH
b) 10.2 mol ethane, C2H6
c) 1.6 × 10−3 mol Na2 SO4
5. Determine the mass in grams of each of the following:
a) 0.600 mol of oxygen atoms
b) 0.600 mol of oxygen molecules, O2
c) 0.600 mol of ozone molecules, O3
6. The Cullinan diamond was the largest natural diamond ever found (January 25, 1905). It weighed 3104 carats (1 carat = 200 mg). How many carbon atoms were present in the stone?
7. A certain nut crunch cereal contains 11.0 grams of sugar (sucrose, C12H22O11) per serving size of 60.0 grams. How many servings of this cereal must be eaten to consume 0.0278 moles of sugar?
8. Which of the following represents the least number of molecules?
a) 20.0 g of H2O (18.02 g/mol)
b) 77.0 g of CH4 (16.06 g/mol)
c) 68.0 g of CaH2 (42.09 g/mol)
d) 100.0 g of N2O (44.02 g/mol)
e) 84.0 g of HF (20.01 g/mol)
9. What is the mass of 4.44 mol of Rb?
10. What is the mass of 12.34 mol of Al2(SO4)3?
Extend
How many moles are present in 236.588 mL of mercury if the density of mercury is 13.6 g/mL? How much would it weigh? How many moles would an equivalent volume of water contain, and what would that weigh?
Answers
1. Formic acid. Its formula has twice as many oxygen atoms as the other two compounds (one each). Therefore, 0.60 mol of formic acid would be equivalent to 1.20 mol of a compound containing a single oxygen atom.
2. The two masses have the same numerical value, but the units are different: The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 × 1023 molecules.
3. a) 197.382 g mol−1 b) 257.162 g mol−1 c) 194.193 g mol−1
4. a) 0.819 g b) 307 g c) 0.23 g
5. a) 9.60 g b) 19.2 g c) 28.8 g
6. 3.113 × 1025 C atoms
7. 0.865 servings, or about 1 serving.
8. 20.0 g H2O represents the least number of molecules since it has the least number of moles.
9. 379 g
10. 4,222 g
Glossary
Avogadro’s number (NA): experimentally determined value of the number of entities comprising 1 mole of substance, equal to 6.022 × 1023 mol−1
formula mass: sum of the average masses for all atoms represented in a chemical formula; for covalent compounds, this is also the molecular mass
molar mass: mass in grams of 1 mole of a substance
mole: amount of substance containing the same number of atoms, molecules, ions, or other entities as the number of atoms in exactly 12 grams of 12C
- Omiatek, Donna M., Amanda J. Bressler, Ann-Sofie Cans, Anne M. Andrews, Michael L. Heien, and Andrew G. Ewing. “The Real Catecholamine Content of Secretory Vesicles in the CNS Revealed by Electrochemical Cytometry.” Scientific Report 3 (2013): 1447, accessed January 14, 2015, doi:10.1038/srep01447. ↵