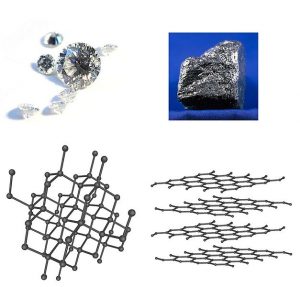
It has long been known that pure carbon occurs in different forms (allotropes) including graphite and diamonds.
Graphite is brittle, whereas diamond is the hardest natural material known on Earth. Yet both are just pure carbon. What is special about this element that makes these two forms of carbon so different?
Bonds. Chemical bonds!
In graphite, each carbon is bonded to three other carbons to form a flat sheets of carbon lattices which are form layers. These layers, called graphene, are attracted to each other through Van der Waals forces, a type of intermolecular force. Graphite is brittle because these intermolecular forces are relatively weak.
In a perfect diamond crystal, each C atom makes four connections—bonds—to four other C atoms in a three-dimensional matrix. Four is the greatest number of bonds that is commonly made by atoms, so C atoms maximize their interactions with other atoms. This three-dimensional array of connections extends throughout the diamond crystal, making it essentially one large molecule. Breaking a diamond means breaking every bond at once.
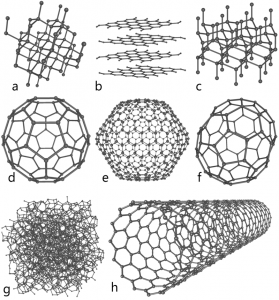
It was not until 1985 that a new form of carbon was recognized: buckminsterfullerene, commonly known as a “buckyball.” This molecule was named after the architect and inventor R. Buckminster Fuller (1895–1983), whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface. Experimental evidence revealed the formula, C60, and then scientists determined how 60 carbon atoms could form one symmetric, stable molecule. They were guided by bonding theory—the topic of this chapter—which explains how individual atoms connect to form more complex structures.