12 Offshore Chemical Engineering & Quantum Technologies For Maritime Risk & Resilience (Sharkey)
What if the smartest system on Earth was not on land or in orbit—but drifting silently beneath the waves? Offshore chemical quantum intelligence is no longer science fiction—it’s strategic infrastructure. -Dr. Sharkey

Figure 12-1: Quantum-enhanced offshore platform at sunset with a female engineer and chemical visualization. Source: Image generated by ChatGPT using OpenAI’s DALL·E, 2025.
Chemical engineering has long been the province of land-based megastructures—industrial ecosystems built for scale, not mobility. However, as operational demand shifts toward autonomous systems, offshore platforms, and distributed energy logistics, chemical manufacturing is beginning to move offshore. Maritime chemical engineering is evolving beyond basic desalination and corrosion prevention into a new class of energy-forward infrastructure that supports onboard fuel synthesis, emission management, and resource independence at sea.
Maritime infrastructure is transforming. The ships, platforms, and support systems that once relied on brute force and analog control are now becoming intelligent, chemically aware, and computationally autonomous. As global risks converge—climate disruption, contested waterways, cyber-physical attacks—the sea is emerging not just as a domain to secure, but as an environment to compute within.
This chapter explores the intersection of offshore chemical engineering (ChemE) and quantum technologies in the maritime domain. It focuses on the use of quantum computing for molecular simulation of CO₂–ammonia systems, the development of alternative fuels such as green ammonia, and the deployment of GPS-independent navigation systems based on quantum gravimetry. These are not speculative prototypes—they are technologies being tested, validated, and in some cases, deployed by defense agencies and research institutions across multiple continents.
Real-world examples include quantum algorithms running on IBM hardware to simulate ammonia capture reactions, autonomous gravimetric navigation systems tested in naval environments, and the emergence of AI-enhanced control systems for offshore fuel synthesis. At their core, these systems are about resilience—operating in isolation, adapting to change, and ensuring continuity of mission in the face of uncertainty.
This chapter continues the thread of interdisciplinary foresight work—extending previous contributions on quantum supply chains in space to the floating laboratories of the ocean. Where space was vacuum and isolation, the sea is pressure and entropy. Yet both domains now require infrastructure that can learn, compute, and chemically respond.
Learning Objectives:
By the end of this chapter, readers will be able to:
- Describe the role of offshore chemical engineering in the maritime domain, with a focus on emissions control, fuel generation, and material resilience.
- Understand how quantum computing is applied to simulate chemical systems such as ammonia–CO₂ capture relevant to marine applications.
- Evaluate the use of quantum sensing and navigation systems as secure, GPS-independent alternatives for defense and autonomous platforms.
- Analyze how chemical, quantum, and AI systems interact to form adaptive maritime infrastructure.
- Assess the operational, strategic, and sustainability implications of these emerging systems within maritime transportation frameworks.
Section 12.1 Chemical Engineering at Sea: Foundations and Strategic Relevance
At the center of this, the maritime chemical engineering shift is ammonia (NH₃)—a molecule composed of one nitrogen atom and three hydrogen atoms. While long used as a fertilizer precursor, ammonia is now emerging as a strategic fuel and energy carrier, particularly for the maritime sector. Its carbon-free combustion profile, high hydrogen density, and existing handling infrastructure make it uniquely suited for long-haul, non-electric vessel propulsion systems.
Historically, the Haber–Bosch process has been the dominant method for synthesizing ammonia by reacting nitrogen gas (N₂) from the atmosphere with hydrogen gas (H₂), typically derived from fossil fuels. [1] [2] [3] (See Endnote [1]). The process proceeds as follows:
N2(g) + 3H2(g) ⟷ 2NH3(g) ΔH = −92.5 kJ/mol Eq 12.1
This reaction occurs under high temperatures (400–500°C) and pressures (150–250 atm) with an iron-based catalyst and has been a cornerstone of industrial chemistry since the early 20th century (Darmawan & Lokahita, 2012). However, it is also highly energy-intensive. Conventional ammonia plants require 26–35 GJ per ton of NH₃ produced, with a theoretical minimum around 20 GJ/ton. Despite optimizations, current best-in-class plants operate in the 27–30 GJ/ton range, while future electrolysis-fed systems could consume up to 55 GJ/ton unless integrated with advanced efficiency strategies (IEA, 2021). As of 2021, U.S. natural gas spot prices and pipeline disruptions continued to influence ammonia production costs, reinforcing the case for decoupling maritime fuel synthesis from volatile fossil markets (U.S. Energy Information Administration, 2021).
Traditionally confined to static, land-based facilities, ammonia production is now being reconsidered for offshore and mobile deployment. Recent advances in modular synthesis reactors, low-pressure designs, and containerized electrolysis units are enabling ammonia production at sea. Electrolysis splits water (H₂O) into hydrogen (H₂) and oxygen (O₂):
2H2O(l) → 2H2(g) + O2(g) Eq. 12.2
This hydrogen, when reacted with nitrogen (N₂) extracted via air separation, allows ammonia to be synthesized without the use of fossil fuels—effectively decarbonizing the input stream and enabling onboard or offshore green fuel production (IEA, 2021; Ning et al., 2023). Global shifts are also reshaping ammonia production strategies, with countries like China—historically, the world’s largest ammonia producer—beginning to diversify away from coal-based feedstocks toward cleaner, modular, and electrolysis-powered systems (Ammonia Energy Association, 2019).
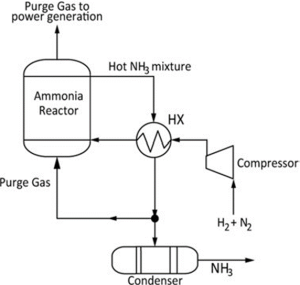
Similarly, methanol (CH₃OH) is gaining traction as a synthetic liquid fuel that can be produced using hydrogen and captured carbon dioxide (CO₂):
CO2(g) + 3H2(g) → CH3OH(l) + H2O(l) Eq. 12.3
This pathway, as explored in marine energy research, enables a partial or closed-loop carbon cycle when integrated with carbon capture systems, such as exhaust gas scrubbers on vessels or direct ocean capture (Mignard & Pritchard, 2006).
These offshore applications of classical chemical synthesis are particularly relevant for mission continuity, fuel autonomy, and platform resilience—key criteria in both defense and commercial shipping scenarios.
Furthermore, the ammonia infrastructure is already partially in place: dedicated ammonia tankers, refrigerated storage systems, and handling procedures from the fertilizer and industrial chemical sectors are now being adapted for maritime bunkering and shipboard fuel delivery (IEA, 2021). As recent infrastructure panel discussions confirm, global port terminals are already piloting ammonia bunkering systems, emphasizing scalable retrofitting of existing fuel logistics to accommodate NH₃ storage and transfer (Ammonia Energy Association, 2021).
In tandem, chemical subsystems continue to play critical roles across maritime platforms, including:
- Corrosion inhibition systems for structural longevity
- Antifouling coatings to reduce drag and bioaccumulation
- Desalination and water treatment systems supported by chemical conditioning
- Carbon capture units based on aqueous ammonia or amine solvents
These systems are being engineered for durability, chemical stability, and autonomous function—designed to operate in variable salinity, high-pressure, low-maintenance environments (Ning et al., 2023).
In addition to electrochemical fuel synthesis, next-generation maritime energy systems are exploring compact nuclear platforms, including floating modular reactors (FMRs) that may be deployed for civilian or strategic applications. These systems are designed to operate independently of terrestrial energy grids and have the potential to support offshore ammonia synthesis, desalination, and electrolysis operations—all while ensuring a reliable energy backbone in isolated marine environments.

Floating reactors may operate on traditional fission cycles or experimental fusion prototypes involving the tritium-deuterium (T–D) reaction, a process in which tritium (³H) and deuterium (²H) nuclei fuse to produce helium-4 (4He) isotope and high-energy neutrons (n) (Popular Science, 2023):
D + T → 4He + n + 17.6 MeV Eq. 12.4
This reaction is one of the most energetically efficient known, but it requires advanced systems to produce, contain, and recycle tritium fuel, often using lithium blankets and chemical isotope separation. Recent engineering advances now make it feasible to incorporate these cycles within hardened maritime platforms, including designs intended to endure tropical storms, thermal cycling, and prolonged autonomous deployment.
The architecture of these systems typically includes:
- Compact reactor cores housed within maritime hulls or subsea modules
- Radiation shielding integrated into the vessel structure
- Onboard desalination and electrolysis units supported by nuclear power
- Smart monitoring systems for predictive maintenance and containment
Floating nuclear platforms offer the possibility of coupling nuclear heat and power with ammonia production loops, enabling continuous synthesis even in low-wind or low-solar zones (Ammonia Energy Association, 2022)—ideal for long-haul shipping lanes, deep-sea research bases, or forward-operating naval installations.
As these designs become more viable, chemical engineering challenges emerge, such as tritium containment, corrosion management in radioactive environments, isotope separation for fuel refinement, and chemical recovery loops that must remain operational with minimal human oversight. These efforts represent a convergence of chemical systems, materials science, and autonomous diagnostics.
In addition to its utility as a synthetic fuel and hydrogen carrier, ammonia may serve as a chemical host for tritium—an isotope critical to both experimental fusion and nuclear deterrence systems. Tritiated ammonia compounds (e.g., ³NH₃ or ND₃) offer a potential medium for compact, chemically bound storage of tritium fuel aboard floating nuclear platforms. This approach may provide safety, containment, and logistical advantages over cryogenic or metallic hydride-based storage systems, particularly in mobile or high-pressure marine environments.
Ammonia’s strategic complexity extends beyond fuel synthesis and nuclear applications. As the precursor to ammonium nitrate (NH₄NO₃), it is also central to the manufacture of civilian and military-grade explosives. Ammonium nitrate-based formulations, such as ANFO, have featured prominently in both terrestrial mining operations and naval armaments, creating dual-use concerns for maritime chemical supply chains. As ammonia infrastructure expands offshore, safety protocols, supply chain monitoring, and chemical conversion oversight will become essential to prevent misuse and ensure alignment with international maritime security norms.
Together with quantum-optimized synthetic fuel generation, compact nuclear power adds another layer of resilience and redundancy to future maritime platforms, supporting multi-vector energy autonomy in a world where strategic independence at sea is increasingly vital.
Finally, while these systems are inherently chemical, their design and optimization increasingly depend on digital technologies—including quantum computing and AI, which allow engineers to simulate reaction pathways, optimize catalyst behavior, and anticipate system failure modes. These tools represent a new computational layer for maritime ChemE, addressed in detail later in this chapter.
As these roles expand, ammonia is no longer just a fuel—it becomes a platform molecule for carbon capture, catalysis, and system-scale transformation, discussed in detail in the next section.
Section 12.2 Ammonia as a Strategic Molecule: Fuel, Capture Medium, and Catalyst
Ammonia has historically been a linchpin of global agriculture, enabling large-scale nitrogen fixation and fertilizer production. Today, it is rapidly evolving into a strategic molecule at the intersection of energy systems, carbon management, and materials chemistry. In offshore and maritime contexts, ammonia is more than a fuel—it is a multi-role agent supporting fuel synthesis, carbon capture, catalytic transformation, and chemical storage, with significant implications for logistics, resilience, and security.
- Fuel and Energy Carrier
As established in Section 12.1, ammonia is gaining prominence as a carbon-free fuel for marine propulsion. Its volumetric energy density, hydrogen content, and liquid-phase handling characteristics make it favorable for long-range operations. However, its role as an energy carrier extends further: NH₃ can store renewable hydrogen in chemical form, transport it across oceans, and be cracked back into hydrogen on demand using onboard or offshore catalytic reactors.
Ammonia’s potential as a universal hydrogen carrier is particularly relevant in maritime trade routes where renewable production (via electrolysis) occurs in resource-rich locations. Still, hydrogen demand is concentrated in industrial or strategic regions. Mobile ammonia systems offer a practical pathway to bridge this spatial and temporal gap.
- Medium for Carbon Capture
In addition to its energy value, ammonia functions as a CO₂ capture solvent, either alone or in aqueous solution. Aqueous ammonia systems can selectively absorb carbon dioxide from exhaust streams in maritime engines or onboard reactors, forming stable ammonium carbamate or carbonate compounds. These products can then be processed to regenerate ammonia and recover concentrated CO₂ for utilization or sequestration.
Recent studies suggest ammonia-based CO₂ scrubbing may outperform traditional amine solvents in marine settings, due to:
- Lower degradation rates under variable thermal loads
- Higher CO₂ loading capacity
- Reduced corrosiveness and lower regeneration energy demands
This makes ammonia a chemically adaptive system component, enabling ships and offshore platforms to implement closed-loop carbon cycles or comply with emission caps without sacrificing range or power.
- Catalyst and Systems Enabler
Ammonia is also a precursor to advanced materials and catalytic agents used in maritime chemical systems. For example:
- Metal–ammonia complexes are explored in low-temperature fuel cells.
- Ammonia-derived intermediates are essential for nitrogen-doped carbon catalysts in electrochemical reactors.
- Ammonia decomposition products can act as in situ reductants or hydrogen donors for methanation, Fischer–Tropsch, or methanol synthesis systems.
Ammonia’s ability to switch roles—as feedstock, reductant, fuel, or capture agent—makes it uniquely suited for multifunctional chemical engineering in constrained environments like ships or offshore reactors. This chemical flexibility allows for dynamic process reconfiguration based on energy availability, mission requirements, or environmental conditions.
Recent developments in direct ammonia fuel cells (DAFCs) offer a compelling path toward low-temperature, high-efficiency onboard power generation. Unlike ammonia cracking methods that require hydrogen liberation, DAFCs use ammonia directly as a fuel, significantly simplifying system architecture. In an alkaline DAFC, the core electrochemical reactions are:
Anode (oxidation):
NH₃ + 3OH⁻ → ½ N₂ + ³⁄₂ H₂O + 3e⁻ Eq. 12.5
Cathode (reduction):
³⁄₂ O₂ + 3H₂O + 3e⁻ → 6OH⁻ Eq. 12.6
Overall reaction:
NH₃ + ³⁄₂ O₂ → ½ N₂ + ³⁄₂ H₂O Eq. 12.7
These reactions highlight the direct use of ammonia without the need for intermediate hydrogen gas, increasing efficiency and reducing system complexity. Advances in hydroxide exchange membrane (HEM) technologies and catalyst design—especially the use of metal complexes based on ruthenium (Ru), platinum (Pt), and palladium (Pd)—have led to improved ammonia oxidation kinetics and reduced activation losses at the anode.
Theoretical work has expanded our understanding of metal–ammonia molecular complexes as tunable platforms for both catalysis and quantum information science. Jackson, Khan, and Miliordos (2023) highlight that these complexes can stabilize unusual oxidation states and electronic configurations due to strong coordination effects with ammonia ligands. In catalysis, these interactions are relevant for ammonia oxidation and decomposition processes, particularly when designing earth-abundant metal alternatives to platinum-group catalysts. Their work also emphasizes the structural flexibility and electron density distributions of expanded metals solvated by ammonia. This could inform the design of selective, low-temperature catalysts for marine fuel cells and reformers.
Research into non-precious metal catalysts (e.g., nickel- and cobalt-based systems) is also ongoing to reduce costs and enable wider adoption. Despite persistent challenges such as ammonia crossover, catalyst poisoning, and system degradation under variable maritime loads, DAFCs represent a promising solution for distributed, zero-carbon energy production at sea (Abbasi et al., 2020). Their compatibility with compact fuel reformers and electrochemical stacks makes them well-suited to hybrid architectures where ammonia serves as both a long-range energy carrier and an immediate source of electricity for mission-critical subsystems.
Recent studies have shown that the performance of oxygen reduction reactions (ORR) in alkaline direct ammonia fuel cells (DAFCs) can be significantly influenced by the chemical structure of the electrocatalyst. Nitrogen-doped carbon (N–C) materials, for instance, offer promising activity in oxygen reduction under alkaline conditions, which is essential for DAFC cathodes. Wan et al. (2015) demonstrated that the electrocatalytic behavior of these N–C catalysts is extremely sensitive to pH, with notable variations in kinetic parameters and half-wave potential across different buffer conditions. This sensitivity underscores the importance of reactor pH control and electrolyte design in maritime fuel cell applications. The presence of graphitic and pyridinic nitrogen moieties in the carbon matrix plays a central role in enhancing catalytic activity—offering a non-precious metal pathway to efficient ORR in harsh marine environments.
Ongoing advances in ammonia decomposition chemistry have opened new possibilities for hydrogen provisioning in fuel cell systems. Rather than oxidizing ammonia directly at the anode, some architectures rely on catalytic decomposition to produce hydrogen and nitrogen:
2NH₃ → N₂ + 3H₂ Eq. 12.8
This endothermic reaction enables integration with proton exchange membrane fuel cells (PEMFCs) by supplying high-purity hydrogen from a dense ammonia feedstock. Transition metals—including ruthenium (Ru), nickel (Ni), cobalt (Co), and iron (Fe)—exhibit promising activity as catalysts, especially when dispersed on high-surface-area supports such as alumina (Yin et al., 2004).
Ammonia decomposition offers logistical and operational advantages in maritime settings, providing a scalable pathway for hydrogen generation that bypasses the need for cryogenic or compressed storage. In mobile or remote offshore environments, this approach enhances flexibility for hybrid fuel cell systems and strengthens the role of ammonia as a multifunctional energy carrier.
The convergence of offshore chemical engineering and advanced materials points to an increasingly complex design space—one where molecular interactions, catalytic behavior, and system performance must be optimized across harsh, dynamic environments. Classical modeling techniques, while effective for many design scenarios, face limitations when simulating multi-electron systems, quantum tunneling effects, or the behavior of strongly correlated materials central to ammonia-based catalysis and carbon capture. To address these challenges, researchers are turning to quantum computing as a powerful tool for simulating chemical reactions at the molecular level. In the next section, we explore how quantum algorithms—implemented on platforms such as IBM Quantum—are enabling precise modeling of CO₂–ammonia interactions, revealing new reaction pathways, optimizing catalysts, and accelerating the development of resilient maritime chemistries.
Section 12.3 Quantum Simulation of Maritime Chemical Systems
As maritime energy systems become more chemically complex, the need for accurate modeling of molecular reactions under operational conditions becomes critical. Traditional computational methods, such as density functional theory (DFT) and classical molecular dynamics, have enabled significant advances in catalyst design and reaction engineering. However, these methods struggle with strongly correlated systems, reaction barriers, and excited-state dynamics relevant to real-world maritime chemistry. In this context, quantum computing offers a powerful alternative—one capable of simulating chemical systems at the quantum mechanical level with scalable precision.
Quantum Simulation of Ammonia and CO₂ Interactions
Recent studies have demonstrated that quantum computers can now be used to model key reactions central to maritime chemical systems, including the interaction between ammonia (NH₃) and carbon dioxide (CO₂). In a foundational investigation by Lee et al. (2024), quantum algorithms such as Variational Quantum Eigensolvers (VQE) and Quantum Imaginary Time Evolution (QITE) were employed to simulate the ground-state energy surfaces and binding energetics of CO₂ capture by NH₃ under varying geometries. The results, validated against high-level classical methods, show that quantum devices can not only match conventional calculations but offer a tractable path to real-time, in-situ chemical prediction for marine systems with minimal human tuning. These calculations account for realistic reaction geometries and dynamic conformations, which are crucial in variable-pressure, offshore environments.
This builds on prior work by Nguyen et al. (2023), who used VQE and quantum coupled-cluster techniques to simulate vibrational modes of the CO₂–NH₃ complex. Their results revealed how hydrogen bonding strength and angular geometries of the ammonia molecule modulate its ability to chemically bind and reduce CO₂—insights that are invaluable for designing maritime CO₂ scrubbers and reactive carbon loops. Notably, they showed that these simulations become more accurate with increased qubit fidelity, underscoring the synergy between hardware development and chemical modeling.
What is a Qubit?
A qubit or quantum bit is the basic unit of quantum information—analogous to a classical bit, but capable of existing in multiple states simultaneously due to the principle of superposition. Unlike classical bits that are either 0 or 1, qubits can be in a state that is both 0 and 1 at once, allowing for dramatically increased computational parallelism. Qubits may also exhibit entanglement, a quantum property enabling instantaneous correlations between particles, regardless of distance.
For a foundational introduction, see “Look Before You Leap: Demystifying Quantum Computing’s Enigmatic Frontier” in Advanced Technologies for Humanity (Nichols et. al., 2024).
The application of quantum computing to chemical simulation is not new—but its growing maturity is now making complex systems, such as ammonia–CO₂ interactions, tractable at useful scales. In 2017, IBM demonstrated a pioneering simulation of beryllium hydride (BeH₂) using its quantum hardware, marking a shift from toy models to more chemically relevant systems (Russell, 2017). This milestone represented an inflection point in the use of quantum algorithms for real-world chemical modeling, laying the groundwork for today’s efforts in simulating ammonia-based reactions relevant to carbon capture, fuel synthesis, and platform resilience at sea.
In Figure 12-6, IBM illustrated how early quantum hardware was applied to simulate molecular electronic structure—a foundational step for understanding ammonia–CO₂ reaction energetics. The molecule studied, beryllium hydride (BeH₂), is used here as a test case for quantum chemistry methods that could be extended to more complex systems like NH₃.
What Are Orbitals?
In quantum chemistry, orbitals describe the spatial distributions and energy levels where electrons are most likely to be found around an atom or molecule. Orbitals are not physical orbits but rather probability regions defined by quantum mechanics. Common types include:
- 1s orbital: A spherical region closest to the nucleus; holds up to two electrons.
- 2s orbital: A larger spherical orbital one energy level above 1s.
- 2p orbitals (e.g., 2pₓ, 2pᵧ, 2p_z): Dumbbell-shaped regions oriented along the x, y, or z axes. These represent higher-energy states and can each hold two electrons of opposite spin.
In this simulation, each qubit represents an occupied or unoccupied orbital state, with paired spins encoded into the qubit structure. This mapping forms the foundation of second quantization, a method by which quantum circuits can simulate fermionic systems like electrons in molecules.
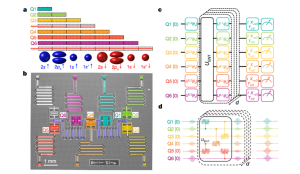
Circuit Overview
Panel (c) displays a variational quantum eigensolver (VQE) circuit, a hybrid algorithm that runs on both classical and quantum hardware. In this layout:
- Single-qubit gates Uᵢʲ(θₖ) perform parameterized rotations.
- The entangler block UENT builds electron correlation by linking qubit states, which is essential to model chemical bonding.
- Measurements are performed on each qubit to extract energy expectation values, which are then minimized using classical optimization.
For a broader introduction to the principles of molecular orbitals, electron correlation, and the fundamentals of quantum chemical simulation, readers are encouraged to consult a book by Dr. Sharkey: Quantum Chemistry and Computing for the Curious (Sharkey, 2022), available at https://www.packtpub.com/en-us/product/quantum-chemistry-and-computing-for-the-curious-9781803238593. This book was listed as one of the 10 Chemistry Books That Separate Experts from Amateurs (Smith, 2025).
Hardware-Level Studies: Nitrogen Activation on Iron Surfaces
A separate but equally critical reaction for ammonia production is the activation and dissociation of nitrogen (N₂)—a notoriously difficult process due to the extreme bond strength of molecular nitrogen (N≡N), one of the strongest known chemical bonds. Using actual quantum hardware, Christopoulou et al. (2024) implemented simulations of nitrogen adsorption and dissociation over iron clusters, a proxy for the Haber–Bosch catalyst surface. These hardware-executed calculations show that real-time reaction barriers and active site behaviors can now be probed experimentally with quantum devices, allowing maritime ammonia systems to be modeled under catalytic operating conditions. Their results demonstrated pathways for optimizing transition states, potentially leading to more efficient offshore ammonia reactors.
Historical Perspective: Ammonia and the Foundations of Quantum Computing
The role of ammonia in quantum technology is not limited to chemistry—it extends into quantum hardware itself. In one of the earliest explorations of ammonia in quantum computing, Ferguson et al. (2002) proposed the use of ammonia inversion states as qubits, leveraging the tunneling between two pyramidal configurations of NH₃ across a potential barrier. Though this concept remains primarily conceptual, it highlights the unique physical characteristics of ammonia as both a molecular substrate and quantum information carrier.
Further supporting the role of ammonia in quantum systems, Bradley (n.d.) highlights the phenomenon of inversion doubling—a quantum mechanical effect where ammonia molecules tunnel between two mirror-image pyramidal configurations separated by a potential barrier. This tunneling results in the splitting of vibrational energy levels into closely spaced doublets, an effect measurable using infrared spectroscopy (~35 cm⁻¹). These inversion states illustrate how quantum behavior emerges from molecular structure, reinforcing why ammonia has been considered for quantum information applications as both a model system and a potential physical qubit candidate.
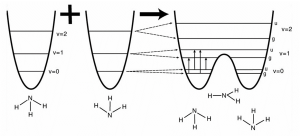
Source: Adapted from Bradley, M. (n.d.). Inversion Doubling of Ammonia. Thermo Fisher Scientific. https://tools.thermofisher.com/content/sfs/brochures/AN50753-E-0415M-Ammonia.pdf
Over the past 20 years, the field has matured considerably. Quantum systems now meet many of the DiVincenzo criteria for practical quantum computation, including scalable qubit fabrication, long coherence times, and gate operations (Georgescu, 2020). In chemical applications, qubit architectures like superconducting circuits, ion traps, and photonic networks are being directly applied to simulate reaction kinetics, enabling the modeling of complex maritime chemical environments with atomistic fidelity.
Strategic Outlook for Offshore Platforms
As offshore systems begin integrating autonomous ammonia reactors, carbon capture loops, and multifunctional fuel cells, quantum computing offers a new axis of control. Future maritime systems may include onboard quantum processors or edge-connected quantum nodes to adaptively simulate chemical conditions— modulating catalyst temperature, reactant flow, or electrochemical setpoints based on predicted molecular behavior. This is particularly vital for mission-critical systems operating without human oversight in dynamic marine environments.
As demonstrated by this growing body of research, quantum computing is not merely an abstract promise for chemistry—it is a maturing capability with direct implications for the resilience, autonomy, and intelligence of maritime infrastructure. As chemical reactions at sea grow increasingly autonomous and computationally governed, so too must the physical navigation systems that support them. In contested maritime environments—where GPS signals can be spoofed, jammed, or entirely unavailable—resilient positioning systems are vital.
Section 12.4 GPS-Free Quantum Navigation Systems for Maritime Defense
Quantum navigation offers an emerging alternative: using the intrinsic properties of matter and gravity to determine position with no reliance on satellites. Drawing on advances in atomic interferometry and gravimetry, these systems enable ships, submarines, and autonomous platforms to calculate their movement through space using fundamental quantum measurements. This section explores the technical basis and strategic relevance of GPS-independent navigation tools, examining how quantum sensors, accelerometers, and AI-enhanced inertial guidance systems are being integrated into the next generation of maritime infrastructure. Where Section 12.3 looked inward—modeling the molecular structure of fuels and reactions—this section looks outward, toward the precise control of movement and location in an increasingly uncertain operational seascape.
Autonomous maritime systems, from unmanned surface vessels (USVs) to forward-deployed sensor buoys, are increasingly vital in securing global waterways. However, these platforms face persistent threats from cyber-physical attacks that can target their navigation and control systems. Traditional Global Positioning System (GPS) guidance is vulnerable to jamming, spoofing, and denial-of-service attacks—making robust, GPS-independent navigation a strategic priority.
As maritime platforms increasingly rely on autonomous systems and AI-driven control, the vulnerability of satellite-based navigation—particularly GPS—to jamming, spoofing, and cyber-attack has become a significant operational concern, and in a 2025 study by Astrov and Bauk, simulations of cyber-intrusion against the rudder control systems of USVs revealed how software-level manipulations of heading data can lead to destabilization or mission failure without physical damage to the platform. These findings underscore the growing demand for secure, inertial, and GPS-independent navigation technologies in both defense and commercial maritime sectors.
One promising solution is quantum gravimetry—a technique that uses quantum sensors to detect subtle changes in gravitational fields for positioning. These systems rely on the quantum properties of atoms (such as rubidium or cesium) placed in free fall within interferometers. The measured phase shift encodes information about local gravitational acceleration, which, when combined with maps of Earth’s gravity, enables inertial positioning without reliance on external signals.
Key advantages of quantum inertial navigation systems (QINS) include:
- Immunity to electromagnetic interference and jamming
- Passive operation—no signal emissions or reception required
- High precision over long durations, especially when hybridized with classical inertial measurement units (IMUs)
In military scenarios, these systems offer critical navigation continuity in contested or denied environments. When integrated into unmanned systems like those studied by Astrov & Bauk, quantum sensors can provide a fail-safe navigation backbone that is resistant to cyber intrusion.
Moreover, coupling QINS with real-time fault detection—potentially via onboard quantum processors or AI systems trained to recognize anomalies in rudder response—can further mitigate the impact of cyber-attacks on vessel behavior.
The convergence of quantum navigation, autonomous control, and cyber-resilience marks a shift in naval architecture. Ships are no longer passive recipients of global signals—they are becoming self-localizing platforms, capable of sustaining operation amid signal loss, spoofing, or attack.
In the context of maritime autonomy and defense resilience, navigation systems must now operate under conditions where GPS signals may be denied, spoofed, or degraded. As ships, drones, and offshore platforms move into contested or remote waters, quantum navigation has emerged as a viable and increasingly validated alternative—offering high-precision positioning without reliance on external satellite infrastructure.
One of the most operationally relevant breakthroughs in this field comes from Q-CTRL, a company recognized for its expertise in quantum control software and hardware optimizatJulyJuly 16y 16 16, 2025, Q-CTRL publicly confirmed the success of its first maritime defense trials, in which a quantum dual gravimeter was installed aboard the Royal Australian Navy’s MV Sycamore, a multi-role training vessel. The system was operational for over 144 hours without human intervention, collecting precise inertial and gravimetric data while at sea.
To understand the operational significance of these systems, it is helpful to consider how quantum gravimeters work briefly. Unlike GPS, which relies on external satellite signals, quantum gravimeters use the interference of matter waves—specifically cold atoms in free fall—to measure tiny variations in gravitational acceleration. These measurements can be used to construct a gravity-based map for localization, immune to spoofing or jamming.
At the core of Q-CTRL’s approach is the concept of quantum gravimetry—a technique that measures infinitesimal variations in Earth’s gravitational field. These variations, or gravity “landmarks,” serve as navigational reference points. Much like traditional orienteering with physical landmarks, this gravimetric map enables vessels to determine their position by comparing real-time gravity readings to pre-measured geophysical data. Because gravity is impervious to electromagnetic interference, gravimetric navigation is immune to spoofing or jamming—unlike GPS.
Zhou et al. (2025) offer a broader review of how quantum gravimetry is now transitioning from laboratory experiments to real-world platforms, highlighting successful demonstrations of portable atom interferometers operating under dynamic conditions. Their findings confirm that performance stability—once a limiting factor for mobile deployment—can now be achieved using advanced laser cooling, vibration compensation, and real-time signal correction. These developments pave the way for gravimetric systems to become core components of autonomous navigation architectures across air, land, and sea domains.
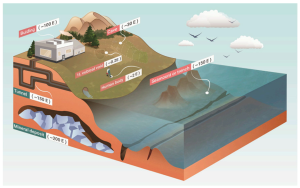
In this deployment, Q-CTRL used a “strapdown” dual gravimeter, compact enough to fit within a server rack and operating at just 180W—about one-tenth the power draw of a standard toaster. The sensor continued functioning even under extreme ship motion and vibration, conditions that would normally disrupt traditional quantum sensing configurations. This resilience was enabled by software ruggedization, Q-CTRL’s proprietary methodology for stabilizing quantum systems outside of controlled laboratory environments.
The strategic implications of these findings are profound. The threat of GPS denial in regions such as the Middle East and Indo-Pacific has become more than theoretical—reports of spoofed signals in maritime choke points are growing, raising the risk of navigational uncertainty, collisions, and lost communication in both civilian and military operations. Q-CTRL’s success indicates that quantum navigation could serve not only as a backup but as a primary navigation method for unmanned or stealth maritime assets operating in high-risk zones.
From a systems design perspective, integrating quantum gravimeters with autonomous guidance, collision avoidance, and mission planning frameworks will likely become a defense priority. Moreover, as adversaries invest in advanced electronic warfare, quantum-assured positioning, navigation, and timing (PNT) systems may serve as a foundational element of future naval doctrine—on par with encrypted communications or hardened propulsion.
Together with prior work by Astrov and Bauk (2025) on simulating cyberattacks on unmanned rudder control systems, these developments highlight the increasing complexity of maritime cyber-physical security. While Astrov’s modeling of adversarial interference at the control system level reveals the vulnerability of actuators and communication buses, Q-CTRL’s gravimetric platform offers an orthogonal mitigation strategy—bypassing vulnerable satellite links altogether.
Quantum navigation does not prevent cyberattacks, but it offers a resilient anchor for localization and timing, upon which secure control and coordination protocols can be built. In that sense, it forms part of a layered maritime defense architecture—combining hardwired inertial sensing, quantum-enhanced signal integrity, and AI-driven fault detection to support operational continuity in environments where classical infrastructure may fail.
Looking ahead, the quantum sensing market is expected to reach $3–5 billion by 2030. Defense platforms will likely be early adopters of maritime quantum navigation, not only for its resistance to interference but also for its ability to support high-value missions such as underwater reconnaissance, fuel rendezvous, and autonomous vessel convoying—without exposing their position to adversarial tracking systems.
In sum, gravimetric quantum sensors offer more than just GPS independence—they offer a step-change in how maritime systems can sense, adapt, and navigate in a contested world. As maritime operations become increasingly autonomous, the value of quantum navigation systems extends beyond mere positioning. These sensors, when integrated with artificial intelligence and inertial navigation systems, unlock a new level of autonomy—enabling vessels to not only know where they are, but to adapt how they move and respond in real time. The fusion of quantum sensing and AI-based control marks the next phase in maritime system design: one where resilience, stealth, and intelligence converge across chemical, navigational, and operational layers. These themes of integrated quantum-AI infrastructure are explored in detail in the following section.
Section 12.5 Integrating Quantum AI in Autonomous Maritime Infrastructure
In modern maritime systems, autonomy is not simply a function of mobility—it’s a function of awareness and adaptation. As platforms become increasingly complex, the integration of artificial intelligence with quantum sensors and control architectures enables dynamic responses to real-time conditions at sea. Whether managing fuel synthesis aboard a floating reactor or navigating under cyber threat without GPS, these systems require not just precision, but prediction.
By integrating AI with quantum-enhanced inertial navigation systems (INS), vessels can perform high-fidelity dead reckoning, detect subtle changes in system behavior, and autonomously reconfigure operations. The stealth and resilience offered by quantum systems—immune to electromagnetic interference and satellite spoofing—combine with AI’s pattern recognition and decision-making capabilities to form the basis of self-governing, mission-adaptive infrastructure.
These hybrid systems can:
- Optimize chemical reaction conditions using live sensor data
- Detect and correct actuator faults before they impact operations
- Manage power allocation across propulsion, life support, and carbon capture subsystems
- Predict and prevent cascading failures through onboard diagnostics and simulation
The following sections explore how these AI-quantum hybrids are enabling resilient chemical processing, adaptive routing, and self-healing capabilities across next-generation maritime platforms.
Integration with AI and Inertial Navigation Systems (INS)
The full potential of quantum navigation is realized when it is integrated into hybrid systems that include artificial intelligence (AI) and traditional inertial navigation systems (INS). Classical INS—based on gyroscopes and accelerometers—can maintain position estimates over short periods, but their accuracy degrades due to drift. Quantum sensors mitigate this limitation by providing absolute references based on physical constants. When fused with AI algorithms, the system can dynamically adjust for sensor noise, detect anomalies, and correct trajectory estimates in real time.
AI-enhanced sensor fusion algorithms are now capable of ingesting quantum gravimetry data alongside classical IMU signals, GPS (if available), and magnetometer inputs to build redundant and resilient navigation stacks. This layered approach not only improves accuracy but also adds fault tolerance. If one subsystem is compromised—by cyberattack, signal loss, or hardware failure—others can compensate based on learned behavioral models and historical patterns.
Machine learning models trained on simulated attack scenarios (as in Astrov & Bauk, 2025) can detect subtle anomalies in control signals or inertial measurements—raising alerts or triggering evasive protocols without human oversight. These AI-enhanced INS frameworks are particularly valuable in unmanned surface vessels (USVs) or underwater autonomous vehicles (UAVs), where communication delays or adversarial jamming make human-in-the-loop navigation infeasible.
Operational Advantages: Stealth, Resilience, and Independence from Satellites
Gravimetric quantum navigation systems confer several strategic benefits that are difficult to replicate with conventional methods:
- Stealth: Because they are entirely passive (they do not emit or receive signals), quantum gravimeters are inherently low observable. This makes them ideal for operations in contested environments where electromagnetic emissions could reveal platform location.
- Resilience: Quantum inertial sensors are immune to common GPS threats such as jamming and spoofing, which are increasingly deployed in global conflict zones and maritime choke points.
- Continuity and Independence: In missions involving long-duration autonomy—deep-sea exploration, undersea cable surveillance, or missile guidance—quantum-enhanced INS systems can maintain accurate navigation without relying on external infrastructure.
Together, these attributes support the design of resilient maritime systems that can operate undetected, unjammed, and uninterrupted, regardless of satellite availability or cyberattack exposure.
As these AI-quantum systems evolve from experimental platforms to deployed technologies, their integration into maritime autonomy becomes not just a possibility—but a necessity. Yet the promise of intelligent, chemically aware vessels capable of navigating, reacting, and self-optimizing in real time comes with significant challenges. Hardware limitations, environmental constraints, and adversarial threats remain persistent barriers. The next section addresses these critical operational risks and readiness concerns, providing a balanced view of what it takes to bring quantum-enhanced maritime systems into the field.
Section 12.6 Risks, Challenges, and Operational Considerations
The deployment of quantum-enhanced systems at sea is no longer a theoretical exercise. From quantum chemical simulation tools that guide ammonia synthesis to gravimetric navigation systems aboard naval vessels, real-world maritime applications are beginning to take shape. However, the transition from laboratory demonstration to operational deployment is fraught with complexity. These systems must contend with:
- Harsh environmental conditions: Temperature fluctuations, vibration, salinity, and mechanical stress in maritime settings can impact sensor fidelity and hardware longevity.
- Cybersecurity threats: As seen in the work of Astrov and Bauk (2025), autonomous navigation systems can be compromised via software-layer attacks. Even AI-enhanced systems are not immune to adversarial interference without proper safeguards.
- SCADA system vulnerabilities: Supervisory Control and Data Acquisition (SCADA) systems used in offshore chemical engineering remain a critical vulnerability point. These centralized control networks manage ammonia reactors, desalination loops, and hydrogen storage units, but are often not designed for adversarial resilience or quantum-era encryption.
Even robust technologies like quantum inertial navigation systems (QINS) must be rigorously tested under prolonged deployment, the timelines for maritime procurement and certification often lag behind technological development. Defense and commercial fleets alike operate on decadal cycles, while quantum hardware evolves on a yearly basis. This mismatch challenges integration planning and raises questions about upgradability, maintenance, and interoperability with legacy chemical engineering systems.
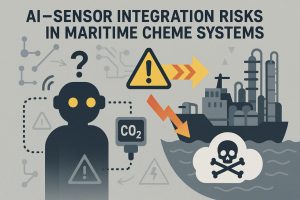
AI–Sensor Integration Risks in Maritime ChemE Systems
As artificial intelligence becomes more tightly integrated with chemical sensors aboard autonomous maritime platforms, a new layer of cyber-physical risk emerges. These AI-enhanced systems, while enabling predictive diagnostics and adaptive control, are vulnerable to sensor spoofing, adversarial data injection, and black-box failures. If an AI system is trained on manipulated or incomplete sensor inputs—such as falsified ammonia levels, pH shifts, or CO₂ gradients—it may misclassify hazards or initiate dangerous responses. This is particularly concerning in autonomous synthesis modules or carbon capture loops, where even minor misreadings can result in runaway reactions or pressure anomalies. Furthermore, AI models often lack explainability, making it difficult for human operators to trace or override faulty logic in time-sensitive scenarios. In contested environments, where electromagnetic interference and cyberattacks are common, sensor networks may also become entry points for adversarial disruption of chemical processes. As chemical platforms evolve into closed-loop, AI-governed systems, they must be designed with multi-layered redundancy, explainable AI logic, and hardened sensor validation frameworks. Failure to do so could expose vessels to cascading system errors, toxic releases, or even adversarial repurposing of onboard chemical infrastructure.
Section 12.7. Future Outlook: Resilient Infrastructure and Floating Intelligence
Maritime infrastructure is no longer a fixed assemblage of hulls, pipelines, and turbines—it is becoming an intelligent, reactive ecosystem. The convergence of quantum sensing, autonomous chemical systems, and AI-driven control is reshaping the sea not just as a space of logistics, but as a computational domain. Offshore platforms are evolving into floating laboratories—environments that not only produce fuels and extract resources but also learn, simulate, and respond to threats in real time.
From Platforms to Platforms-as-Systems
Where once chemical systems were passive and isolated, future offshore units will behave as “platforms-as-systems”—integrated environments that dynamically optimize synthesis, energy distribution, and resilience under variable loads. This will include:
- Onboard quantum processors simulating catalytic behavior and reaction pathways in situ
- AI-coordinated fuel cycles that adapt to sensor data, maintenance schedules, and environmental inputs
- Quantum inertial navigation enabling stealth and continuity in denied environments
- Chemical systems capable of auto-reconfiguring processes in response to energy constraints or mission objectives
This reactive infrastructure is not limited to a single vessel. It extends across distributed maritime networks: semi-autonomous ammonia tankers, unmanned surveillance buoys with onboard gravimeters, and deep-sea research nodes functioning as chemical AI testbeds. These nodes form an oceanic mesh—a modular, mobile grid of intelligence and production capacity.
As outlined in industry foresight by the Boston Consulting Group (2023), quantum sensing is no longer a speculative defense concept, but a maturing technology with commercial applications projected across navigation, exploration, and process optimization. The firm forecasts a $7 billion quantum sensing market by 2030, with maritime deployments—especially for gravimetry and inertial navigation—among the most viable short-term use cases. These capabilities are seen not just as strategic advantages, but as essential components of future offshore resilience.
Strategic Autonomy Beyond Shorelines
In this framework, maritime systems are decoupled from terrestrial dependencies. Instead of waiting for resupply, maintenance, or satellite updates, next-generation offshore infrastructure will be able to generate its energy, simulate its chemistry, and navigate by physics alone.
Such autonomy is critical in geopolitical environments where contested waterways and cyber threats dominate. Nations that control floating chemical intelligence—that is, mobile, computationally aware fuel and sensing platforms—will have a strategic edge in logistics, deterrence, and response.
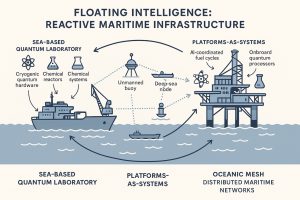
Strategic Autonomy Beyond Shorelines
This autonomy does not mean isolation; rather, it enables coordination without constant supervision. Platforms can share data with satellites, relay analytics to command systems, and integrate with terrestrial supply chains when secure—but remain operational when those links are compromised.
The Rise of Sea-Based Quantum Laboratories
One foreseeable outcome is the development of sea-based quantum laboratories—vessels or oceanic facilities equipped with:
- Cryogenic quantum hardware housed in vibration-dampened maritime environments
- Chemical reactors linked to real-time quantum simulation modules
- Gravimetric mapping arrays for deep-sea mineral prospecting and navigation
- AI-driven fault detection tools for structural and process health monitoring
These laboratories could serve dual roles: conducting fundamental research and supporting applied missions such as fuel generation, ocean monitoring, or defense analytics. In effect, the ocean becomes a testbed for post-classical technologies, from photonic entanglement networks to self-organizing AI systems that evolve their chemical architectures.
Ethics, Governance, and Risk Frontiers
With this intelligence comes risk. As autonomous platforms gain decision-making power over chemical processes, navigation, and system defense, governance frameworks must evolve. Key considerations include:
- Fail-safes for AI-chemical interactions that could pose ecological or kinetic hazards
- Oversight protocols for algorithmic updates and model retraining in remote environments
- Rules of engagement for autonomous vessels in contested waters
- Cyber-physical auditing tools to prevent manipulation of gravimetric or catalytic systems
Moreover, chemical AI models will need transparency: their decision logic, risk thresholds, and failure modes must be auditable—not just by engineers, but by multidisciplinary governance teams.
Conclusions: Toward a Chemically Cognizant Ocean
What emerges is not merely smarter infrastructure—it is a chemically cognizant ocean, one where reaction dynamics, energy flows, and navigational decisions are part of a shared, sensing, adaptive system. This vision of floating intelligence aligns with larger shifts in planetary computation, wherein physical domains (sea, space, atmosphere) are embedded with reactive knowledge systems.
In the maritime domain, these shifts will define the next generation of resilience:
- Resilience is not through redundancy, but through anticipation.
- Resilience is not through mass, but through intelligence.
- Resilience is not bound by shoreline but distributed across waves.
The ocean is no longer a frontier to conquer—it is becoming a collaborator in computation, chemistry, and control.
References:
Abbasi, R., Setzler, B. P., Wang, J., Zhao, Y., Wang, T., Gottesfeld, S., & Yan, Y. (2020). Low-temperature direct ammonia fuel cells: Recent developments and remaining challenges. Current Opinion in Electrochemistry, 21, 335-344.
Advanced Technologies for Humanity: Nichols, Randall, Ackerman, Patricia, DeMaio, Doug, Knaple, Brent, Larson, Haley, McCreight, Robert, Mumm, Hans, Murthy, Rajesh, Sharkey, Keeper, Ryan, Julie: 9798334513129: Amazon.com: Books. (2025). Amazon.com. https://www.amazon.com/Advanced-Technologies-Humanity-Randall-Nichols/dp/B0DC3SW3JT
Ammonia as an Alternative Maritime Fuel.December 5cember 5. Ammonia Energy Association. https://ammoniaenergy.org/presentations/ammonia-as-alternative-maritime-fuel/
Ammonia in China: change is coming.November 7vember 7. Ammonia Energy Association. https://ammoniaenergy.org/articles/ammonia-in-china-change-is-coming/
Ammonia infrastructure: panel wrap-up from the 2020 Ammonia Energy Conference.January 22nuary 22). Ammonia Energy Association. https://ammoniaenergy.org/articles/ammonia-infrastructure/
Ammonia production from offshore nuclear power. April 144 April 14. Ammonia Energy Association. https://ammoniaenergy.org/articles/ammonia-production-from-offshore-nuclear-power/
Astrov, I., & Bauk, S. (2025). Simulating Cyber-Attacks on the Unmanned Sea-Surface Vessel’s Rudder Controller. In Maritime Cybersecurity (pp. 83-102). Cham: Springer Nature Switzerland.
Bradley, M. (n.d.). Inversion Doubling of Ammonia. ReJulyJuly 31y 31 31, 2025, from https://tools.thermofisher.com/content/sfs/brochures/AN50753-E-0415M-Ammonia.pdf
Figure 12-11: Chlorine Poison Gas Attack Ypres 1915, Source: https://d.ibtimes.co.uk/en/full/1434594/poison-gas.jpg
Christopoulou, G., Di Paola, C., Elzinga, F. E., Jallat, A., Ramo, D. M., & Krompiec, M. (2024). Quantum hardware calculations of the activation and dissociation of nitrogen on iron clusters and surfaces. Physical Chemistry Chemical Physics, 26(7), 5895-5906.
Darmawan, A., & Lokahita, B. (2012). Haber-Bosch Process – an overview. Sciencedirect.com. https://www.sciencedirect.com/topics/engineering/haber-bosch-process
Ferguson, A., Cain, P.A., Williams, D.A., Briggs, G.A.D. (2002). Ammonia-based quantum computer. Physical Review A, 65(3), 034303. https://doi.org/10.1103/PhysRevA.65.034303
Georgescu, I. (2020). The DiVincenzo criteria 20 years on. Nature Reviews. Physics, 2(12), 666-666.
IEA. (2021). Executive Summary – Ammonia Technology Roadmap – Analysis. IEA. https://www.iea.org/reports/ammonia-technology-roadmap/executive-summary
Jackson, B. A., Khan, S. N., & Miliordos, E. (2023). A fresh perspective on metal ammonia molecular complexes and expanded metals: opportunities in catalysis and quantum information. Chemical Communications, 59(71), 10572-10587.
Lee, Y. L., Nguyen, M. T., Alfonso, D., Shao, Q., Avramidis, B., Paudel, H. P., … Y Duan, Y. (2024). Assessing Ground State Energy of Molecules and Energy Profile of the NH3 Capturing CO2 System Using the Quantum Computing Algorithms. National Energy Technology Laboratory (NETL), Pittsburgh, PA, Morgantown, WV, and Albany, OR (United States).
Lin, J. (2017, August 19). These are China’s plans for floating nuclear reactors. Popular Science. https://www.popsci.com/china-floating-nuclear-reactors/
Making Sense of Quantum Sensing. July 177 July 17. BCG Global. https://www.bcg.com/publications/2023/making-sense-of-quantum-sensing
Mignard, D., & Pritchard, C. (2006). Processes for the synthesis of liquid fuels from CO2 and marine energy. Chemical Engineering Research and Design, 84(9), 828-836.
Natural Gas Weekly Update. April 11 April 1). Www.eia.gov. https://www.eia.gov/naturalgas/weekly/archivenew_ngwu/2021/04_01/
Nichols, R. K.. Chlorine and Fritz Haber – The Monster February 5bruary 5, 2022) https://www.linkedin.com/feed/update/urn:li:ugcPost:6895811228661612544/
Ning, Y., Wang, L., Yu, X., & Li, J. (2023). Recent developments in the decarbonization of marine and offshore engineering systems. Ocean Engineering, 280, 114883.
Nitrogen-doped Graphitic Porous Carbon (NGPC), 1g. (2025). Acsmaterial.com. https://www.acsmaterial.com/nitrogen-doped-graphitic-porous-carbon.html?srsltid=AfmBOorI-nRyfbmvWXWN1F3css_ekkc3262EDdNByVi0w96BVUjAVMWb
Nguyen, M. T., Lee, Y. L., Alfonso, D., Shao, Q., & Duan, Y. (2023). Description of reaction and vibrational energetics of CO2–NH3 interaction using quantum computing algorithms. AVS Quantum Science, 5(1).
Figure 12-9: OpenAI. (2025). AI-Driven Chemical Infrastructure Risks [Digital illustration]. DALL·E.
Figure 12-10: OpenAI. (2025). Floating Intelligence: Reactive Maritime Infrastructure [Digital illustration]. DALL·E.
Figure 12-2: OpenAI. (2025). Quantum-enhanced offshore platform at sunset, with female engineer and chemical visualization [Digital illustration]. OpenAI DALL·E.
Figure 12-4: OpenAI. (2025). Offshore ammonia synthesis and CO₂-to-fuel conversion platform [Digital illustration]. OpenAI.
Q-CTRL. July 166 July 16) Q-CTRL’s New Maritime Quantum Navigation Solution Successfully Undergoes First Defense Trials at Sea. Q-Ctrl.com; Q-CTRL. https://q-ctrl.com/blog/q-ctrls-new-maritime-quantum-navigation-solution-successfully-undergoes-first-defense-trials-at-sea
Russell, J.September 14ember 14). IBM Breaks Ground for Complex Quantum Chemistry. HPCwire. https://www.hpcwire.com/2017/09/14/ibm-breaks-ground-complex-quantum-chemistry/
Sharkey, K. L., Chance, A., & Khan, A. (2022). Quantum Chemistry and Computing for the Curious. Packt Publishing Ltd.
Smith, D., Sosnic, T., & Hill, G. (June 28, 28, 28). 10 Chemistry Books That Separate Experts from Amateurs [Review of 10 Chemistry Books That Separate Experts from Amateurs]. https://Bookauthority.org/; Book Authority. https://bookauthority.org/books/best-chemistry-books
Wan, K., Yu, Z. P., Li, X. H., Liu, M. Y., Yang, G., Piao, J. H., & Liang, Z. X. (2015). pH effect on electrochemistry of nitrogen-doped carbon catalyst for oxygen reduction reaction. Acs Catalysis, 5(7), 4325-4332.
Zhou, L., Zhong, J., Tang, B., Chen, X., & Zhou, L. (2025). Quantum gravimetry is going toward real applications. The Innovation, 3(3), -. https://www.the-innovation.org/article/id/648ae23ecf340000a100298f
Endnotes
[1] CHLORINE AND FRITZ HABER – THE MONSTER WITHIN by Senior Professor Randall K. Nichols (Article in Li 5bruary 5, 2022
My youngest daughter is studying the periodic table via Great Courses© (Davis, 2022). Somehow, the old chemical engineer was dragged back into the study group. The topic was Chlorine, natural gas with atomic number 17 and atomic weight 35.45 (period 3 and group 17 on the famous Dmitri Mendeleev Table). (Rao & Rao, 2015) Chlorine was discovered by Carl Wilhelm Steel in 1774 while investigating a reaction of manganese dioxide and hydrochloric acid. It was elevated to the status of an element by Humphry Davy, who noted the pale green color from the Greek chloros. Chlorine is a nonmetallic element that prefers to accept an electron to become a negatively charged ion. It is abundant in seawater- the taste comes from the sea of ions. It is a natural balance to the metals dissolved in the ocean, including gold. Chlorine is a very active and remarkably dense elemental gas – so dense that a sample of chlorine molecules sinks in the air – a property exploited by one of the most famous (or infamous) chemists of the 20th century, German Fritz Haber. (Davis, 2022)
The First-Ever Chlorine Gas Attack
Haber is remembered for designing chemical weapons before/during WWI and overseeing the release of 5,730 large pressure tanks containing 168 tons of chlorineApril 2222 April 1915, as part of German Operation Disinfection in the Ypres Sector of Belgium near the North Sea. The prevailing wind was from the west, shifted to the North, where the Canadian and French troops were in battle with German soldiers. (Walker, 2022) Death was not instantaneous, according to a Canadian soldier witness, A. T. Hunter:
“It reached the parapet, paused, gathered itself like a wave, and ponderously lapped over into the trenches. Passive curiosity turned into active torment – a burning sensation in the head, red-hot needles in the lungs, the throat seized by a strangler. Many fell and died on the spot. Others, gasping, stumbled with faces contorted, hands wildly gesticulating, and uttering horse cries of pain, fled madly through the villages and farms and Ypres itself, carrying panic to the civilian population and filling the roads with fugitives of both sexes and all ages.” (Walker, 2022)

Fritz Haber
Haber wasn’t always a monster. At the University of Karlsruhe, he experimented with applying high temperature and pressure to chemical processes. Circa 1905, Haber created a process to produce ammonia from thin air. Combining hydrogen and nitrogen from the atmosphere, Haber’s process produced huge amounts of ammonia fertilizer to boost the yield of almost every crop in the world! (Walker, 2022) In 1911, he joined the industrial giant BASF to commercialize his work. He became wealthy by producing ammonia in Germany and licensing plants in other countries. Haber, a Jewish chemist, rose to the apex of the scientific community. [2] [3]
In 1911, Haber was named to lead the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry in Berlin. Haber was tasked to increase the weapons of Germany and explosives for artillery shells. He perfected a process to convert ammonia into nitric acid, which could be transformed into sodium nitrate for explosives. Experts have estimated that without Haber’s work, a shortage of explosives would have forced Germany to surrender in 1916, two years before the armistice.
Metamorphosis of the Soul
One of the new weapons Haber perfected was poison gas. He claimed that his primary goal was not to kill but to induce panic in the enemies’ ranks.
“ Every new weapon is capable of winning a war.” He wrote. “Every war is a war against the soul of a soldier, not the body.”
Haber spent most of his time at his Berlin institute, exploring the production of deadlier poisons, developing more-efficient delivery systems, and designing gas masks for German troops. He also developed a cyanide-based pesticide that could eliminate mice in granaries and lice in bunkers. Haber called it ZYKLON. He delivered phosgene gas to the German General Staff, a powerful asphyxiant, and mustard gas, which burned the skin and eyes and cauterized the lungs. He perfected their delivery in artillery shells. (Walker, 2022)
Nobel Prize
In 1911, Haber was awarded the Nobel Prize in Chemistry based on his humanitarian work in agriculture. There were quite a few protests to no avail.
[2] Haber traveled to the Eastern Front 2 days after Clara’s death. From a bunker, he wrote a friend:
“For a month, I doubted I could keep going. But now the war with its dreadful images and constant demands on all my powers has made me calmer… It really does me good, every few days, to be at the front, where the bullets fly.”
[3] Earth’s oceans contain ~ 321 billion cubic miles of seawater, each containing 38 pounds of dissolved gold.
