Solutions to Chapter 3 Exercises
3.1 Solutions
1. The spectrum consists of colored lines, at least one of which (probably the brightest) is red.
3. 3.15 m
5. 3.233 × 10−19 J; 2.018 eV
7. ν = 4.568 × 1014 s; λ = 656.3 nm; Energy mol−1 = 1.823 × 105 J mol−1; red
9. (a) λ = 8.69 × 10−7 m; E = 2.29 × 10−19 J; (b) λ = 4.59 × 10−7 m; E = 4.33 × 10−19 J; The color of (a) is red; (b) is blue.
11. E = 9.502 × 10−15 J; ν = 1.434 × 1019 s−1
13. Red: 660 nm; 4.54 × 1014 Hz; 3.01 × 10−19 J. Green: 520 nm; 5.77 × 1014 Hz; 3.82 × 10−19 J. Blue: 440 nm; 6.81 × 1014 Hz; 4.51 × 10−19 J. Somewhat different numbers are also possible.
15. 5.49 × 1014 s−1; no
3.2 Solutions
1. Quantized energy means that the electrons can possess only certain discrete energy values; values between those quantized values are not permitted.
3.
5. −8.716 × 10−18 J
7. −3.405 × 10−20 J
9. 33.9 Å
11. 1.471 × 10−17 J
13. Both involve a relatively heavy nucleus with electrons moving around it, although strictly speaking, the Bohr model works only for one-electron atoms or ions. According to classical mechanics, the Rutherford model predicts a miniature “solar system” with electrons moving about the nucleus in circular or elliptical orbits that are confined to planes. If the requirements of classical electromagnetic theory that electrons in such orbits would emit electromagnetic radiation are ignored, such atoms would be stable, having constant energy and angular momentum, but would not emit any visible light (contrary to observation). If classical electromagnetic theory is applied, then the Rutherford atom would emit electromagnetic radiation of continually increasing frequency (contrary to the observed discrete spectra), thereby losing energy until the atom collapsed in an absurdly short time (contrary to the observed long-term stability of atoms). The Bohr model retains the classical mechanics view of circular orbits confined to planes having constant energy and angular momentum, but restricts these to quantized values dependent on a single quantum number, n. The orbiting electron in Bohr’s model is assumed not to emit any electromagnetic radiation while moving about the nucleus in its stationary orbits, but the atom can emit or absorb electromagnetic radiation when the electron changes from one orbit to another. Because of the quantized orbits, such “quantum jumps” will produce discrete spectra, in agreement with observations.
3.3 Solutions
1. For example, Na+: 1s22s22p6;
Ca2+: 1s22s22p6;
Sn2+: 1s22s22p63s23p63d104s24p64d105s2;
F–: 1s22s22p6; O2–: 1s22s22p6;
Cl–: 1s22s22p63s23p6.
3. (a) 1s22s22p3;
(b) 1s22s22p63s23p2;
(c) 1s22s22p63s23p64s23d6;
(d) 1s22s22p63s23p64s23d104p65s24d105p4;
(e) 1s22s22p63s23p64s23d104p65s24d105p66s24f9
5. The charge on the ion.
(a)
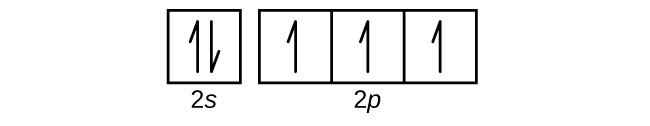
(b)
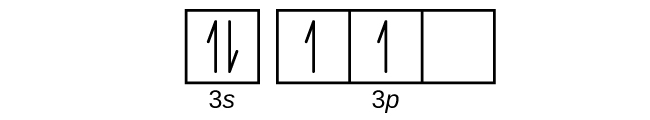
(c)
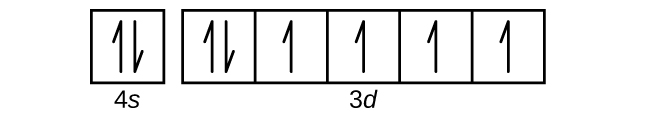
(d)
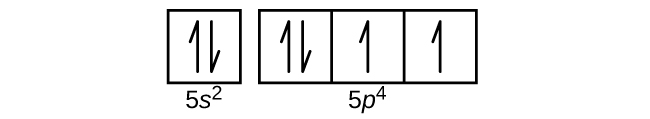
(e)
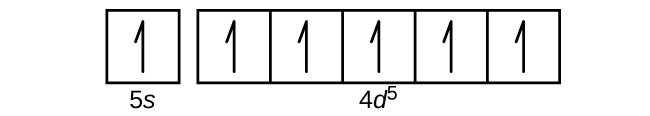
9. Zr
11. Rb+, Se2−
13. Although both (b) and (c) are correct, (e) encompasses both and is the best answer.
15. K
17. 1s22s22p63s23p63d104s24p64d105s25p66s24f145d10
19. Co has 27 protons, 27 electrons, and 33 neutrons: 1s22s22p63s23p64s23d7.
I has 53 protons, 53 electrons, and 78 neutrons: 1s22s22p63s23p63d104s24p64d105s25p5.
