48 Outcome 3: Electron Theory of Electricity
Outcome/Competency: You will be able to escribe the nature of electricity in terms of matter and waves
Rationale:
Why is it important for you to learn this skill?
Your business is electricity. Understanding how electricity flows through powerlines will not only help you determine if you have done your job correctly, it may save your life. You will learn why electricity flows easily through some things, and less easily through others. It is essential to your safety that electricity does not flow through your hotstick, for example.
Objectives:
To be competent in this area, the individual must be able to:
- Define and apply the terms, laws and sources related to the study of electricity
- Describe the relationship between electricity and magnetism
Learning Goals
- Describe electricity in terms of matter and atomic structure
- Rationalize why a material makes a good conductor or insulator
- Describe, identify, and apply laws of electricity to natural phenomena
- Describe the sources of electricity and requirements to induce voltage and current
Introduction:
This chapter will review how electricity is defined in terms of matter and atomic structure, how certain materials are better used as a conductor or insulator, how laws of electricity apply to natural phenomena, and the requirements for electricity to induce voltage and current. This chapter will include class discussions, articles to be reviewed, and opportunities to practice throughout.
Topic 1: Law of Attraction and Repulsion
Attraction and Repulsion
About 2500 years ago, the Greeks discovered that amber rubbed by cloth attracted feathers and other small fibers. The Greek name for amber is “electron.”
The Greek meaning for the word electric is “to be like amber,” or have the property of attraction.
Around 300 years ago, scientists discovered that a piece of charged glass would attract some charged objects and repel others. They learned that the force of repulsion was as common as the force of attraction.
From experiments with different charged materials, two lists were developed.
|
List A |
List B |
|
Glass (rubbed on silk) |
Hard rubber (rubbed on wool) |
|
Glass (rubbed on wool or cotton) |
Block of sulfur (rubbed on wool of fur) |
|
Mica (rubbed on cloth) |
Most kinds of rubber (rubbed on cloth) |
|
Asbestos (rubbed on cloth or paper) |
Sealing wax (rubbed on silk, wool or fur) |
|
Stick of sealing wax (rubbed on wool) |
Glass or mica (rubbed on dry wool) |
|
|
Amber (rubbed on cloth) |
It was discovered that:
- Materials in List A and List B are attracted to each other,
- Materials in List A repel other materials in List A, and
- Materials in List B repel other materials in List B.
Upon the completion of the experiment, scientists decided to call each material in List A “positive charge” and the materials in List B “negative charge.”
With this, the Law of Attraction and Repulsion was stated as:
Law of Attraction and Repulsion
Opposite Charges Attract and Like Charges Repel
Topic 2: Structure of an Atom
Structure of an Atom
All matter is composed of atoms. Atoms are composed of three particles.
- Electrons are the lightest particle (negative charge.)
- Neutrons are small, dense particles (no charge.)
- Protons are small, heavy particles (positive charge.)
Protons and neutrons combine to form the nucleus of the atom.
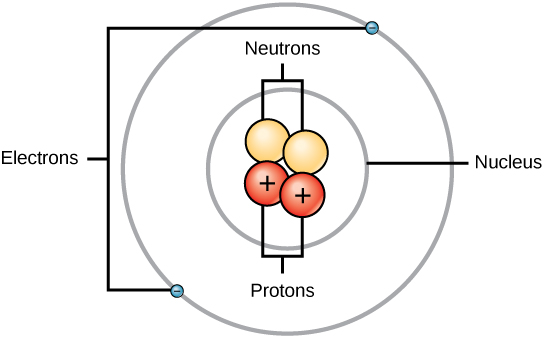
This Photo by Unknown Author is licensed under CC BY-SA
The sun or central sphere is referred to as the nucleus. The planets revolving around it are referred to as electrons. The orbiting electron has a negative charge (-), while the positively charged proton (+) remains stationary in the middle. You can also see that neutrons make up the nucleus but have no charge and no effect on the proton, hence the name neutron.There can be any number of neutrons in the nucleus; however, depending on the atom, protons and electrons are always equal in number. Since the nucleus carries a positive charge and electrons carry a negative charge, we can see that an atom is held together by the forces of attraction. Electrons orbit the nucleus in a series of orbits. In a simple hydrogen atom, there is only one orbit. A more complex atom such as copper, has so many electrons, they must spread into four orbits.
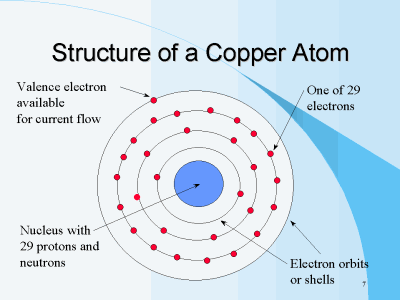
This Photo by Unknown Author is licensed under CC BY-SA
The last (furthest) orbit in any atom is called the “valence shell” or “valence ring;” therefore, the electrons in these rings are called “valence electrons.”One electron in the valence shell makes copper a good conductor.
|
It should be noted that this is a simplified version of the modern understanding of the structure of an atom. In reality, electrons exist around the nucleus in three dimensional spheres and move at almost the speed of light. For our purposes; however, the simplified model will be sufficient. For more information regarding the modern understanding of the structure of the atom, check out the website: https://www.britannica.com/science/atom/Atomic-bonds
|
Review Exercises: Structure of an Atom
Topic 3: Conductors and Insulators
In some materials, the valance electrons are attracted to the nucleus much less than the electrons in the inner rings or orbits.
These valence electrons can be forced from the atom entirely. They are now called “free electrons.”
Once an electron has left its atoms valence ring, the atom is left with a deficiency of electrons, thereby developing a positive charge. When that electron attaches itself to another atom’s orbit, the surplus of electrons develops a negative charge. The difference in charge causes the electrons to flow or move from atom to atom. This flow is called electric current.
Conductors
Conductors are generally made of materials with one or two valence electrons.
These materials allow electrons to flow through them with little effort. A domino effect occurs in conductors as electrons as electrons hump from their orbit to adjacent atoms, which in turn, forces the electrons out of their orbit. This continues from atom to atom along the length of the conductor.
The ease at which these electrons move through material determines the efficiency of the conductor. Some examples of good conductors are:
- Silver (best, but too expensive)
- Copper (almost as good)
- Aluminum (good conductor and economical)
- Salt is an excellent conductor
Insulators
Since a good conductor has few valence electrons, an insulator must have many valence electrons, thus creating a stable and tightly bound orbit.
In reality, there is no such thing as a “perfect” insulator, there are only poor conductors.
An insulator’s valence ring will have seven or eight valence electrons. This valence ring is very stable and does not give up electrons easily.
Insulators are extremely important as they keep electrons from traveling to places we do not want them to. Without insulators, the transportation and utilization of electricity would be impossible, as you can well imagine.
Some materials with seven or eight valence electrons are:
- Porcelain
- Rubber
- Wood
Practice Exercises
Topic 4: Sources of Electricity
4.1 Sources of Electricity
Electrons can be excited to move by several methods: there are a number of ways of producing electricity.
Static (Frictional Energy)
Electricity can be produced by rubbing certain dissimilar materials together. This causes the separation of charges and the objects being rubbed assume opposite charges. These charges are stationary (nonmoving) and are not use in the production of electricity. Static charges are usually more of a nuisance than anything, but also have useful applications. For example, removal of smoke particles in industrial plants, or photocopying and electrostatic painting.
Mechanical Pressure (Piezoelectricity)
Some materials develop opposite charges on opposite sides when compressed, bent, twisted or stretched. For example, the bending of a quartz crystal can cause an alternating voltage to be produced on its faces. This type of electrical production is used in:
- Crystal pick-ups for stereos and microphones
- The starter on a gas barbeque or some lighters.
Chemical Energy
An example of chemical energy is a battery. A battery works by the chemical reaction that takes place between the cell electrodes and the fluid electrolyte. The electrodes (2) connected to the battery terminal react with the electrolyte (acid) and, in tur, form opposite charges. This is the fundamental basis of the operation of a battery.
Light
A beam of light causes certain materials to change automatically which, in turn, changes the electron movement in the materials. When light is striking the photosensitive material, the electrons flow. Electricity can be produced by this method and is quite commonly used in solar cells (calculators or solar panels.)
This production method of electricity should not be confused with the photo-eye on streetlights. A photo-eye is a “photo-conductive” device which does not produce electricity but changes its resistance when struck by light.
Heat
If a copper wire is heated on one end, electrons will flow to the other end which is cooler. In lead or iron, however, the opposite is true (electrons will flow to the warmer end.) So, if you connect these two conductors, twist the ends together and heat them, current will flow.
An example of this is the thermocouple in a furnace. When the pilot light is on, it heats the thermocouple, and the resulting current holds the safety valve open with an electromagnet. If the pilot light goes out, the thermocouple ceases to produce current and the valve closes, preventing gas escaping.
4.2 Magnetism (Electromagnetic Induction)
This is the most common method of electricity production. Almost all power we use in our everyday lives originally comes from a generator in a power plant which operates on the magnetic principle. The generator is literally drawn by either water (hydro plant) or steam (heated by coal, gas, or atomic power.) The power is produced because of the action between the wires and the magnets inside the generator.
In some instances, we use generators that rotate conductors through a magnetic field, in other cases we rotate a magnetic field around a conductor. As long as there is relative motion, current will be produced.
If you were to move a wire across a magnet or vice versa, you would produce a flow of electrons. If you had a meter connected to the conductor as you moved it across the magnetic field, you would see a deflection in the needle of the meter. However, if you were to hold the wire stationary in the magnetic field, there would only be the initial current flow as the wire was moved into the field. Therefore, we can assume that to produce electricity in a magnetic field, we need relative motion. Therefore, to provide a constant current flow, we would need to move the wire or the magnet back and forth constantly.
There are three requirements to induce a voltage:
- Magnetic field,
- Conductor, and
- Relative motion between the two.
If we increase the magnetic strength, add more length to the conductor, or increase the speed at which the magnetic field is cut, the voltage is increased.
A voltage can be induced in a conductor, even though it is not a closed circuit, the same way voltage is present in an open circuit.
4.3 Induced Current
As with voltage, current can also be induced in a circuit, but with one difference. We have learned current will only flow in a closed circuit, so for current to be induced, we must have a conductor in a closed circuit.
The three requirements to induce a current are the same as for voltage:
- Magnetic field,
- Conductor, and
- Relative motion between the two.
We know that voltage is necessary to push or cause electrons to flow. We can see that any time we have an induced current, and induced voltage will also be present.
It should be mentioned that we must have physical movement between the conductor and the magnetic field in a DC circuit to induce voltage and current. In AC circuits, we must also have the same requirements to induce voltage and current, but we do not have to move the conductor or magnetic field physically. An AC electromagnetic field expands and collapses continuously (DC fields do not). (AC fields alternate directions.) This is the relative motion necessary to induce voltage or current.
4.4 Magnetic and Electromagnetic Fields
There may be some confusion between a magnetic field and an electromagnetic field because they are closely related.
- A magnetic field is produced off a permanent magnet.
- An electromagnet field is produced when a current flows through a conductor and creates a magnetic field. (A magnetic field caused by electricity, hence electromagnetic field.)
To understand how we utilize current and magnetic fields, we must describe how either one can be increased to suit our needs.
To increase current flow, we must do one or more of the following:
- Increase relative motion: If we pass conductors through a magnetic field at a higher speed, higher current results. (Speed up the generator.)
- Increase the turns of conductor: the higher the number of conductors cutting the magnetic field, the higher the current.
- Increase the strength of the magnetic field: if we have a stronger magnetic field, there are more lines of flux for the conductor to cut; therefore, current increases.
On the other hand, we can make an electromagnetic field stronger, such as the case with an electromagnet by:
- Increasing the number of turns (length of conductor) in a coil increases the field length.
- Increasing the current increases the field strength.
- Adding a more permeable core (iron) to the coil increases the density of the magnetic field.
Permeability is the ease at which a substance can be magnetized. Using air, steel, and iron as examples, with the same magnetizing force applied, air will accept one line of magnetic force (flux), steel will accept 1,000, and iron will accept 1,500 lines of flux. This means iron is 1,500 times more permeable than air, and one and a half times more permeable than steel.
4.5 Lenz’s Law
We know that current and voltage can be induced in a conductor, provided there is relative motion and a magnetic field.
There is also one more force at work: self-induction. This is described by Lenz’s Law:
An induced voltage or current will oppose the motion that produced it.
In other words, an induced current flow produces a magnetic field. This magnetic field will oppose the current flow that created it.
4.6 Mutual Induction
We know that when a current flows through a conductor, an electromagnetic field is formed around the conductor. This electromagnetic field:
- Flows around the conductor in flux lines
- Is caused every time a current carrying conductor creates a magnetic field.
On a power line, these flux lines are very large and con cover a large area. If these flux lines pass over another conductor which is grounded and holds no charge, electromagnetic induction occurs. Electromagnetic induction id the reverse of a current carrying conductor creating a magnetic field. In this case, an electromagnetic field causes current to flow in a conductor (must be a closed circuit.)
This is one example of mutual induction, where one conductor’s electromagnetic field produces a voltage or current in an adjacent conductor. It must be noted that this is an example of AC mutual induction, as there is only the expanding and collapsing electromagnetic field supplying relative motion. We study this example because it is very common and dangerous in the line trade.
Practice Exercises
[h5p id=”15″]
