When reading the chemical and biochemical literature, you are likely to encounter several different conventions for drawing molecules in three dimensions, depending on the context of the discussion. While organic chemists prefer to use the dashed/solid wedge convention to show stereochemistry, biochemists often use drawings called Fischer projections and Haworth projections to discuss and compare the structure of sugar molecules.
Fisher projections show sugars in their open chain form. In a Fischer projection, the carbon atoms of a sugar molecule are connected vertically by solid lines, while carbon-oxygen and carbon-hydrogen bonds are shown horizontally. Stereochemical information is conveyed by a simple rule: vertical bonds point into the plane of the page, while horizontal bonds point out of the page.
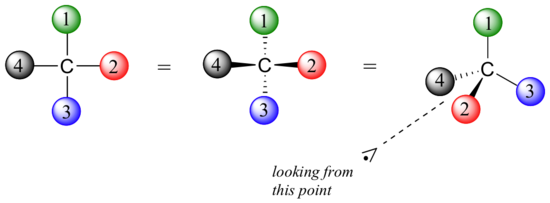
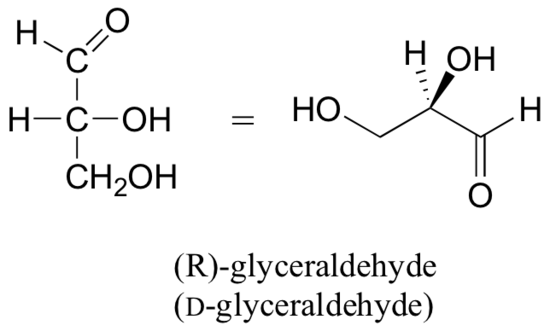
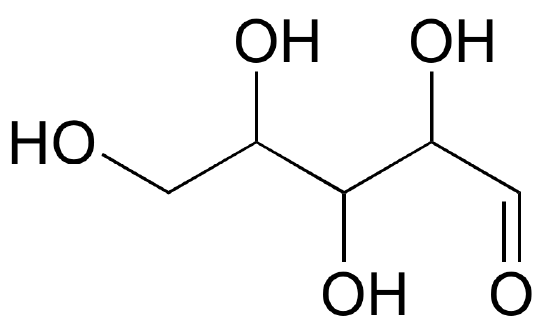
Below are two different representations of (R)-glyceraldehyde, the smallest sugar molecule (also called D-glyceraldehyde in the stereochemical nomenclature used for sugars):
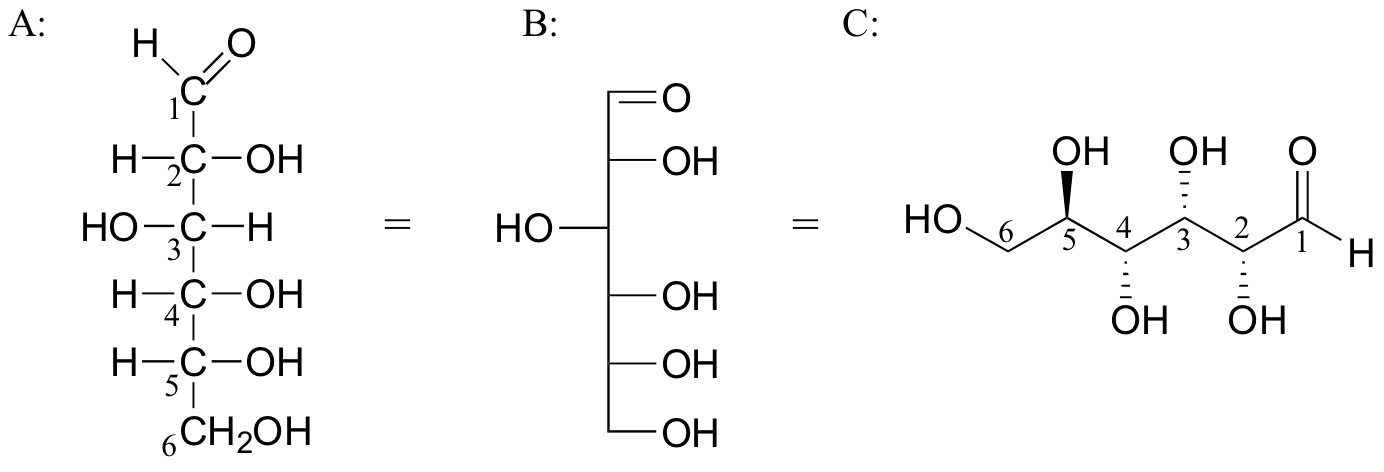
Below are three representations of the open chain form of D-glucose: in the conventional Fischer projection (A), in the “line structure” variation of the Fischer projection in which carbons and hydrogens are not shown (B), and finally in the ‘zigzag’ style (C) that is preferred by organic chemists.
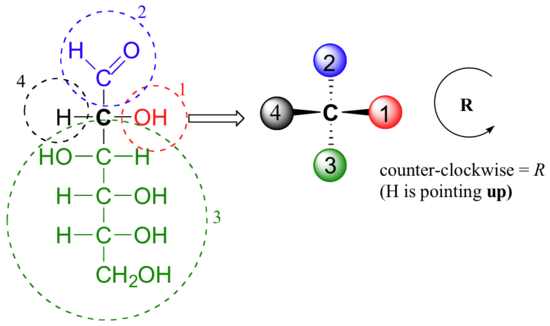
Care must be taken when ‘translating’ Fischer projection structures into’ zigzag’ format – it is easy to get the stereochemistry wrong. Probably the best way to make a translation is to simply assign R/S configurations to each stereocenter, and proceed from there. When deciding whether a stereocenter in a Fischer projection is R or S, realize that the hydrogen, in a horizontal bond, is pointing towards you – therefore, a counterclockwise circle means R, and a clockwise circle means S (the opposite of when the hydrogen is pointing away from you).
Fischer projections are useful when looking at many different diastereomeric sugar structures, because the eye can quickly pick out stereochemical differences according to whether a hydroxyl group is on the left or right side of the structure.
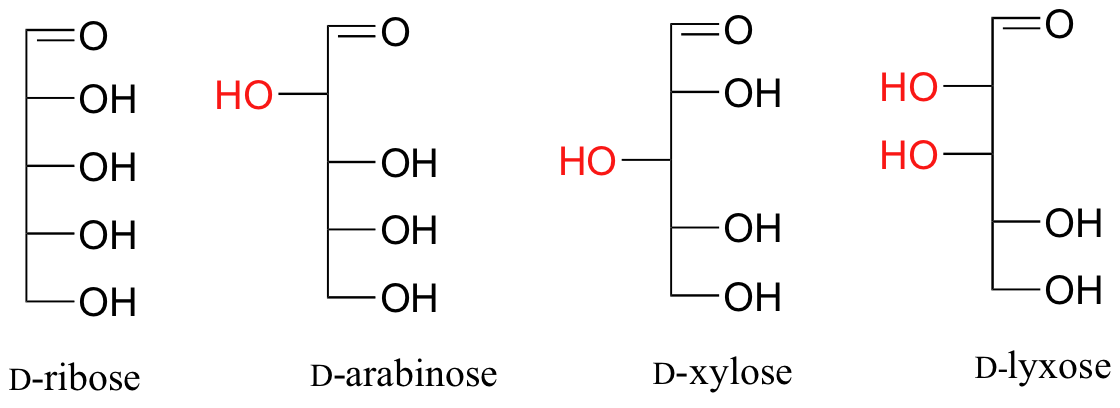
Exercise 3.24: Draw ‘zigzag’ structures (using the solid/dash wedge convention to show stereochemistry) for the four sugars in the figure above. Label all stereocenters R or S. To make it easy to check your answers, draw your structures using the framework below.
While Fischer projections are used for sugars in their open-chain form, Haworth projections are often used to depict sugars in their cyclic forms. The betadiastereomer of the cyclic form of glucose is shown below in three different depictions, with the Haworth projection in the middle.
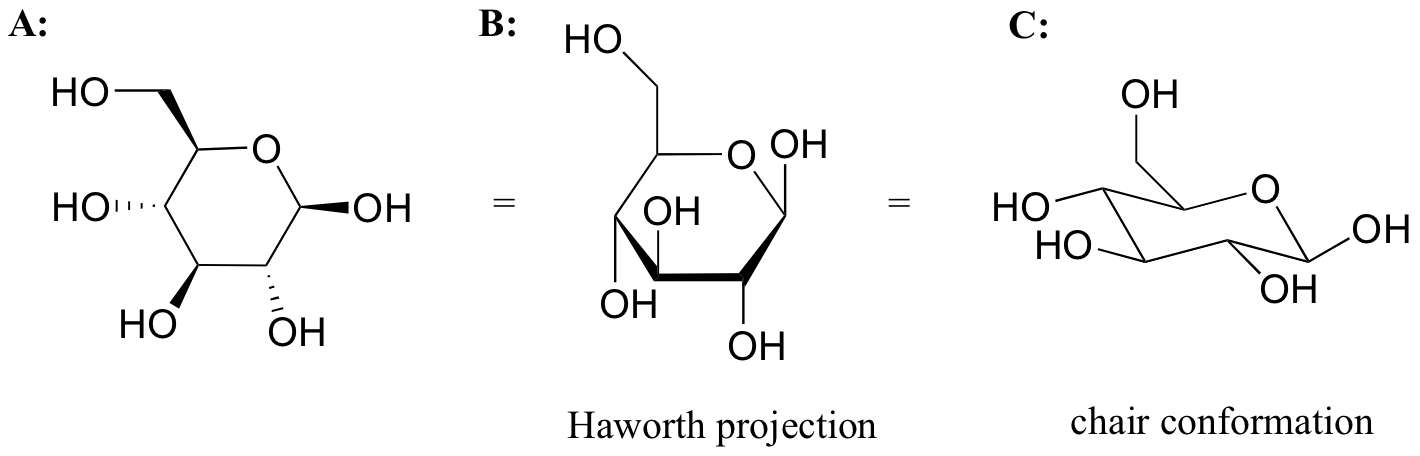
Notice that although a Haworth projection is a convenient way to show stereochemistry, it does not provide a realistic depiction of conformation. To show both conformation and stereochemistry, you must draw the ring in the chair form, as in structure C above.
