Using the ‘line structure’ convention
If you look ahead at the way organic compounds are drawn in following chapters, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in previous chapters. In some cases, you will see condensed structures for smaller molecules instead of full Lewis structures, such as these:
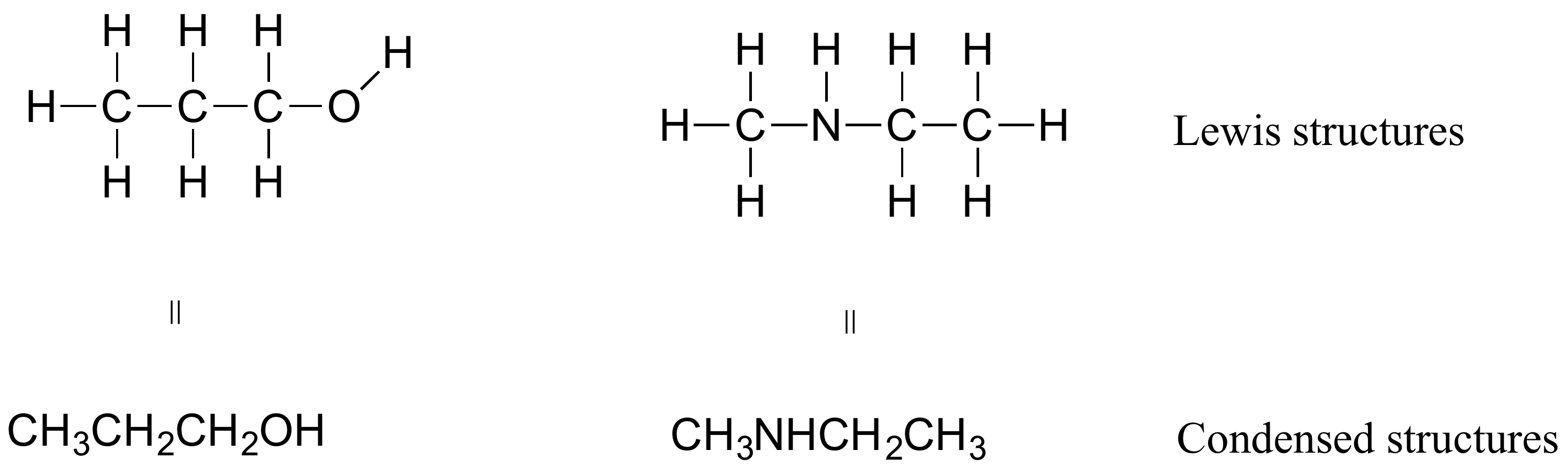
More commonly, organic and biological chemists use an abbreviated drawing convention called line structures. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. Carbon atoms are depicted not by a capital C, but by a ‘corner’ between two bonds or a free end of a bond. Open-chain molecules are usually drawn out in a ‘zig-zig’ shape. Hydrogens attached to carbons are generally not shown: rather, like lone pairs, they are simply implied (unless a positive formal charge is shown, all carbons are assumed to have a full octet of valence electrons). Hydrogens bonded to nitrogen, oxygen, sulfur, or anything other than carbon are shown, but are usually drawn without showing the bond. The following examples illustrate the convention.
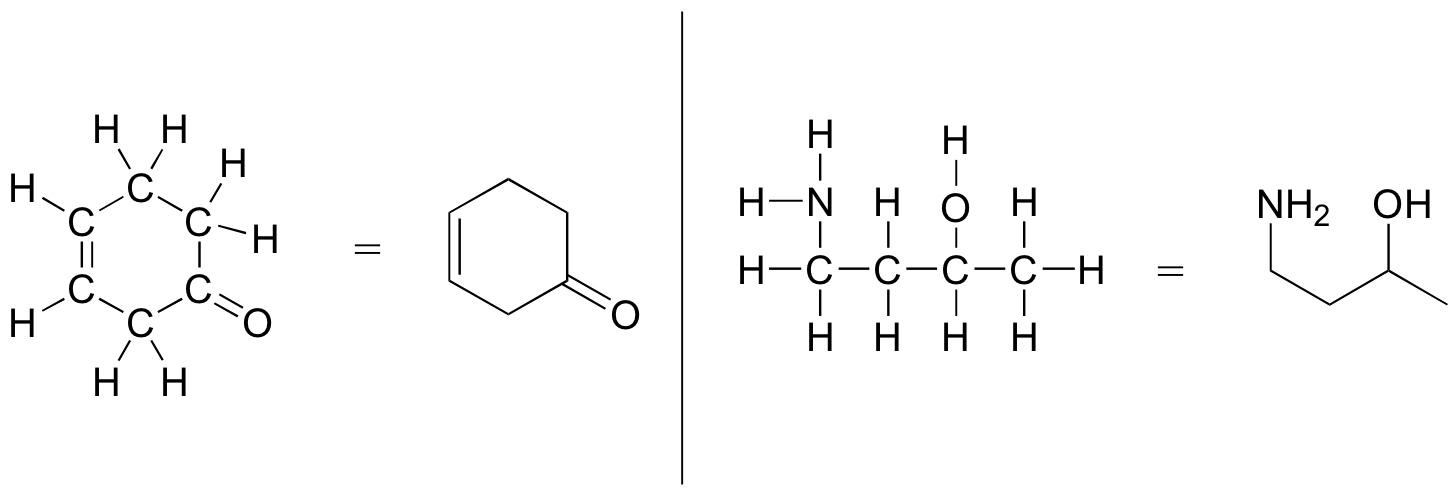
As you can see, the ‘pared down’ line structure makes it much easier to see the basic structure of the molecule and the locations where there is something other than C-C and C-H single bonds. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures.
Sometimes, one or more carbon atoms in a line structure will be depicted with a capital C, if doing so makes an explanation easier to follow. If you label a carbon with a C, you also must draw in the hydrogens for that carbon.
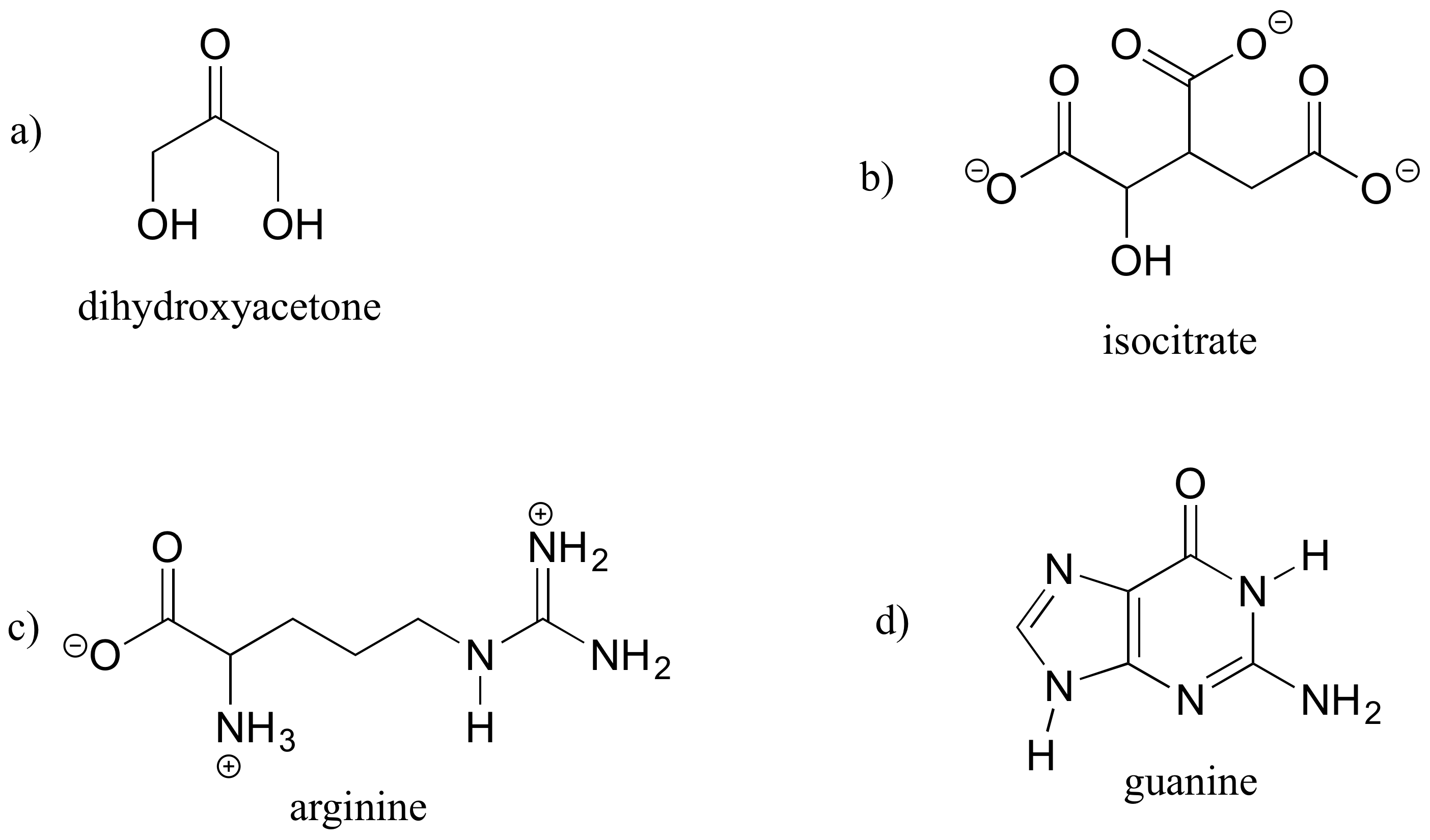
A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Do this for the structures below.
Check Your Learning
Example 1
a) Draw a line structure for the DNA base 2-deoxycytidine (the full structure was shown earlier)
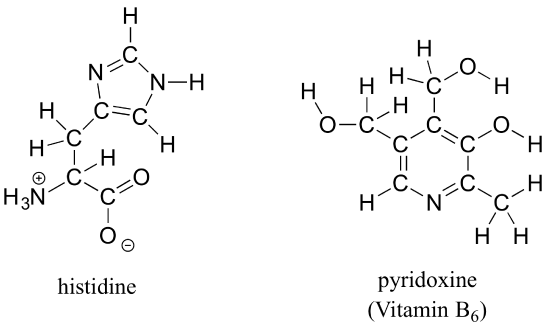
b) Draw line structures for histidine (an amino acid) and pyridoxine (Vitamin B6).
Example 2
Add non-zero formal charges to the structural drawing below:
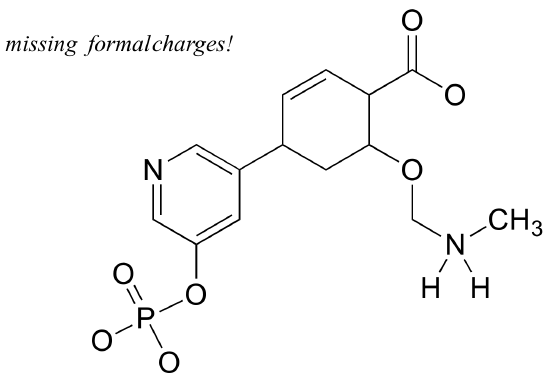
Example 3
Find, anywhere in this textbook, one example of each of the common bonding patterns specified below. Check your answers with your instructor or tutor.
a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge
b) oxygen with two single bonds, two lone pairs, and zero formal charge
c) oxygen with one double bond, two lone pairs, and zero formal charge
d) nitrogen with one double bond, two single bonds, and a +1 formal charge
e) oxygen with one single bond, three lone pairs, and a negative formal charge
