Derived from Foundations of Introductory Chemistry-1 by
Condensation Reactions
A condensation reaction is a reaction in which two molecules combine to form a single molecule. A small molecule, often water, is usually removed during a condensation reaction.
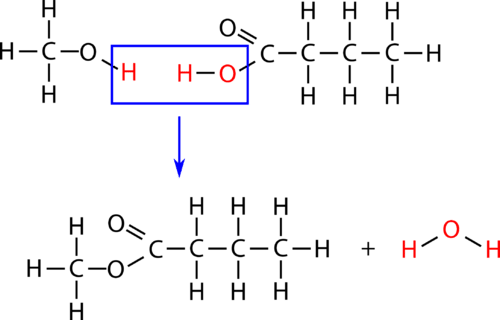
An esterification is a condensation reaction in which an ester is formed from an alcohol and a carboxylic acid. Esterification is a subcategory of condensation reactions because a water molecule is produced in the reaction. The reaction is catalyzed by a strong acid, usually sulfuric acid. When the carboxylic acid butanoic acid is heated with an excess of methanol and a few drops of sulfuric acid, the ester methyl butanoate is produced. Methyl butanoate has the scent of pineapples. The reaction is shown below with both molecular and structural formulas.
Saponification describes the alkaline hydrolysis reaction of an ester. The term saponification originally described the hydrolysis of long-chain esters called fatty acid esters to produce soap molecules, which are the salts of fatty acids. One such soap molecule is sodium stearate, formed from the hydrolysis of ethyl stearate.
ethyl stearate sodium hydroxide sodium stearate (soap) ethanol
The sodium hydroxide is not acting as a catalyst, but is consumed in the reaction.
Amidation reactions
Amino acids are important biological molecules that have an amine functional group on one end of the molecule and a carboxylic acid functional group on the other end. When two amino acids combine in a condensation reaction, a covalent bond forms between the amine nitrogen of one amino acid and the carboxyl carbon of the second amino acid. A molecule of water is then removed as a secondary product.
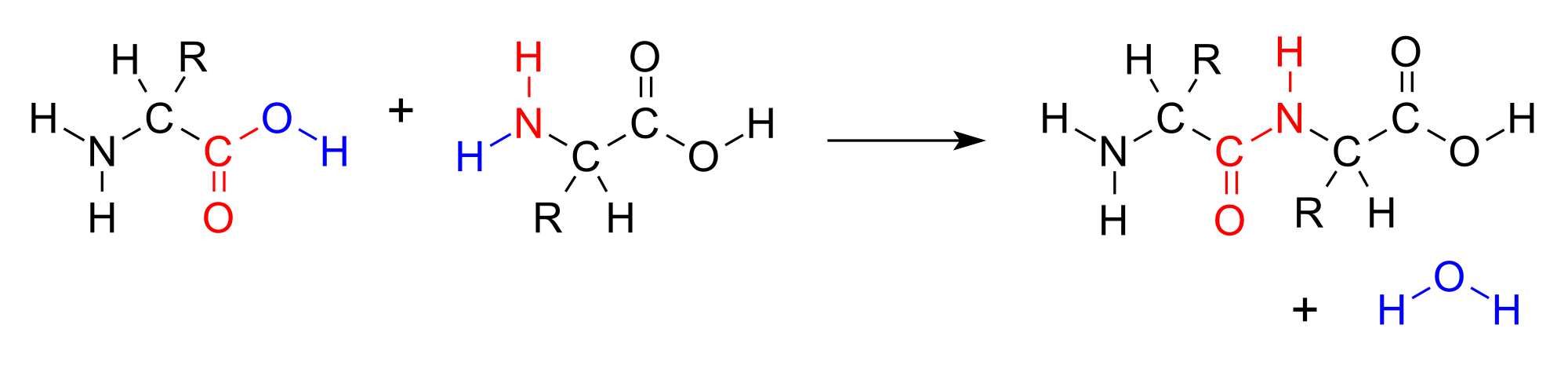 Amino acids join together to form a molecule called a dipeptide. The <span id="MathJax-Element-1-Frame" class="MathJax" style="font-style: normal;font-weight: normal;line-height: normal;font-size: 16px;text-indent: 0px;text-align: left;text-transform: none;letter-spacing: normal;float: none;direction: ltr;max-width: none;max-height: none;min-width: 0px;min-height: 0px;border: 0px;padding: 0px;margin: 0px" role="presentation" data-mathml="">−OH from the carboxylic acid group of one amino acid combines with a hydrogen atom from the amine group of the other amino acid to produce water (blue).
Amino acids join together to form a molecule called a dipeptide. The <span id="MathJax-Element-1-Frame" class="MathJax" style="font-style: normal;font-weight: normal;line-height: normal;font-size: 16px;text-indent: 0px;text-align: left;text-transform: none;letter-spacing: normal;float: none;direction: ltr;max-width: none;max-height: none;min-width: 0px;min-height: 0px;border: 0px;padding: 0px;margin: 0px" role="presentation" data-mathml="">−OH from the carboxylic acid group of one amino acid combines with a hydrogen atom from the amine group of the other amino acid to produce water (blue).This reaction forms a molecule called a dipeptide and the carbon-nitrogen covalent bond is called a peptide bond. When repeated numerous times, a lengthy molecule called a protein is eventually produced.
Polymerization - Addition Polymers
We enjoy the benefits of Styrofoam containers, but don't often think about where they end up. Styrofoam materials do not break down quickly under exposure to the elements. When buried in a landfill, styrofoam will remain intact for a long time. The good news is that there is not a lot of this pollutant found in landfills (maybe about <span id="MathJax-Element-1-Frame" class="MathJax" style="font-style: normal;font-weight: normal;line-height: normal;font-size: 17.6px;text-indent: 0px;text-align: left;text-transform: none;letter-spacing: normal;float: none;direction: ltr;max-width: none;max-height: none;min-width: 0px;min-height: 0px;border: 0px;padding: 0px;margin: 0px" role="presentation" data-mathml="">0.5% by weight of the total mass of garbage). There is no good way to recycle Styrofoam at present, but someday a creative scientist may come up with one.
Polymers are very different from the other kinds of organic molecules that you have seen so far. Whereas other compounds are of relatively low molar mass, polymers are giant molecules of very high molar mass. Polymers are the primary components of all sorts of plastics and related compounds. A polymer is a large molecule formed of many smaller molecules covalently bonded in a repeating pattern. The small molecules which make up the polymer are called monomers. Polymers generally form either from an addition reaction or a condensation reaction.
An addition polymer is a polymer formed by chain addition reactions between monomers that contain a double bond. Molecules of ethene can polymerize with each other under the right conditions to form the polymer called polyethylene.

Polyethylene can have different properties depending on the length of the polymer chains and on how efficiently they pack together. Some common products made from different forms of polyethylene include plastic bottles, plastic bags, and harder plastic objects such as milk crates.
Several other kinds of unsaturated monomers can be polymerized and are components in common household products. Polypropylene is stiffer than polyethylene and is in plastic utensils and some other types of containers.

Polystyrene is used in insulation and in molded items such as coffee cups.

Polyvinyl chloride (PVC) is extensively used for plumbing pipes.

Polyisoprene is a polymer of isoprene and is better known as rubber. It is produced naturally by rubber trees, but several variants have been developed which demonstrate improvements on the properties of natural rubber.

Kevlar (Figure 2) is a synthetic polymer made from two monomers 1,4-phenylene-diamine and terephthaloyl chloride (Kevlar is a registered trademark of DuPont). Kevlar’s first commercial use was as a replacement for steel in racing tires. Kevlar is typically spun into ropes or fibers. The material has a high tensile strength-to-weight ratio (it is about 5 times stronger than an equal weight of steel), making it useful for many applications from bicycle tires to sails to body armor.

The material owes much of its strength to hydrogen bonds between polymer chains (refer back to the chapter on intermolecular interactions). These bonds form between the carbonyl group oxygen atom (which has a partial negative charge due to oxygen’s electronegativity) on one monomer and the partially positively charged hydrogen atom in the N–H bond of an adjacent monomer in the polymer structure (see dashed line in Figure 3). There is additional strength derived from the interaction between the unhybridized p orbitals in the six-membered rings, called aromatic stacking.

Kevlar may be best known as a component of body armor, combat helmets, and face masks. Since the 1980s, the US military has used Kevlar as a component of the PASGT (personal armor system for ground troops) helmet and vest. Kevlar is also used to protect armored fighting vehicles and aircraft carriers. Civilian applications include protective gear for emergency service personnel such as body armor for police officers and heat-resistant clothing for fire fighters. Kevlar based clothing is considerably lighter and thinner than equivalent gear made from other materials (Figure 4).

In addition to its better-known uses, Kevlar is also often used in cryogenics for its very low thermal conductivity (along with its high strength). Kevlar maintains its high strength when cooled to the temperature of liquid nitrogen (–196 °C).