3 3.2 Light and Pigments
Lisa Bartee; Walter Shriner; and Catherine Creech
How Pigments Are Used to Capture Light
How can light be used to make food? It is easy to think of light as something that exists and allows living organisms, such as humans, to see, but light is a form of energy. Like all energy, light can travel, change form, and be harnessed to do work. In the case of photosynthesis, light energy is transformed into chemical energy, which autotrophs use to build carbohydrate molecules. However, autotrophs only use a specific component of sunlight (Figure 1).

What Is Light Energy?
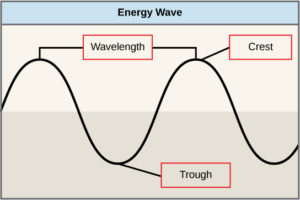
The sun emits an enormous amount of electromagnetic radiation (solar energy). Humans can see only a fraction of this energy, which is referred to as “visible light.” The manner in which solar energy travels can be described and measured as waves. Scientists can determine the amount of energy of a wave by measuring its wavelength, the distance between two consecutive, similar points in a series of waves, such as from crest to crest or trough to trough (Figure 2).
Visible light constitutes only one of many types of electromagnetic radiation emitted from the sun. The electromagnetic spectrum is the range of all possible wavelengths of radiation (Figure 3). Each wavelength corresponds to a different amount of energy carried.
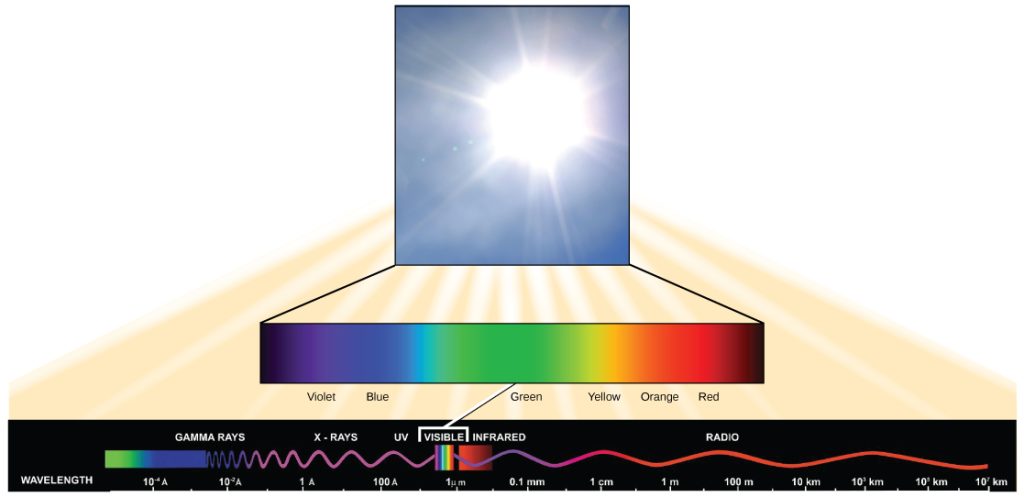
Each type of electromagnetic radiation has a characteristic range of wavelengths. The longer the wavelength (or the more stretched out it appears), the less energy is carried. Short, tight waves carry the most energy. This may seem illogical, but think of it in terms of a piece of moving rope. It takes little effort by a person to move a rope in long, wide waves. To make a rope move in short, tight waves, a person would need to apply significantly more energy.
The sun emits a broad range of electromagnetic radiation, including X-rays and ultraviolet (UV) rays (Figure 3) . The higher-energy waves are dangerous to living things; for example, X-rays and UV rays can be harmful to humans.
Absorption of Light
Light energy enters the process of photosynthesis when pigments absorb the light. In plants, pigment molecules absorb only visible light for photosynthesis. The visible light seen by humans as white light actually exists in a rainbow of colors. Certain objects, such as a prism or a drop of water, disperse white light to reveal these colors to the human eye. The visible light portion of the electromagnetic spectrum is perceived by the human eye as a rainbow of colors, with violet and blue having shorter wavelengths and, therefore, higher energy. At the other end of the spectrum toward red, the wavelengths are longer and have lower energy.
The wavelengths of light that are reflected from an object and bounce off are detected by our eyes. The wavelengths of light that are absorbed by an object do not make it to our eyes. This means that the color an object appears is due to the wavelengths that are reflected and not those that are absorbed. For example, the apple in Figure 4 appears red (assuming you are not color-blind). This is because the red wavelengths of light are reflected off the apple and the other wavelengths (yellow, green, blue, purple) are absorbed by the apple.

Understanding Pigments
Different kinds of pigments exist, and each absorbs only certain wavelengths (colors) of visible light. Pigments reflect the color of the wavelengths that they cannot absorb. All photosynthetic organisms contain a pigment called chlorophyll a, which humans see as the common green color associated with plants. Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but not from green. Because green is reflected, chlorophyll appears green.
Other pigment types include chlorophyll b (which absorbs blue and red-orange light) and the carotenoids. Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light, which is its absorption spectrum.
Many photosynthetic organisms have a mixture of pigments; between them, the organism can absorb energy from a wider range of visible-light wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity decreases with depth, and certain wavelengths are absorbed by the water. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any bit of light that comes through, because the taller trees block most of the sunlight (Figure 5).

[h5p id=”80″]
[h5p id=”81″]
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10
