58 Health and Safety Communication and Documentation
Learning Objectives
- Summarize workplace situations
- Execute documentation
This chapter explains the importance of documents and record keeping. It also shows how they differ and recommends the best approaches for developing written programs.
Standards, policies, and procedures are written in simple, clear language to facilitate record keeping. Written instructions are very useful and assists in your understanding and learning. This leads to employees bringing up good questions to help continually improve a system!
Good documentation lets you quickly double check their own work, without necessarily having to rely on others. Documentation will:
- Prove that programs are effective and being completed as written.
- Demonstrate due diligence.
- Meet requirements for third party customer assessments/audits.
- Meet regulatory requirements; and
- Establish a paper trail to improve the current food safety program
Documents and Records
It is important to understand the difference between a document and a record.
Documents are:
- Permanent
- Describe facility policies and work instructions (Level 1, 2, and 3)
- Define systems, processes, and procedures
Records are:
- Filled in as activity occurs (Level 4)
- Provide proof that policies were followed, or activities performed
- Demonstrate processes and procedures are being conducted as required
Records help provide proof that staff members complete necessary activities and that these activities are performed effectively. Patterns or trends observed in records can be used as a basis for future food safety decisions.
Attempts to falsify records are easily detected. Auditors are trained to look for signs of fraud that can include records completed in the same increasingly messy handwriting and using the same pen. Checking records regularly helps ensure that employees are completing their assigned activities. It helps to make sure that records are being filled out honestly and with all the information needed. Records are an important tool for analyzing and improving food safety. False records will not help improve the system or help you reach your goal of improved food safety!
HACCP records
HACCP plans provide the documents and records needed to make sure that the HACCP system is being followed at each critical control point. HACCP records differ slightly from prerequisite program records. HACCP records provide a historical report of the following:
- Process
- Monitoring procedures
- Deviations; and
- Corrective actions taken at each critical control point (CCP).
These records can take a variety of forms (e.g. processing charts, checklists, written records, computerized records, etc.). HACCP records can help to trace a product or troubleshoot a problem. It’s critical that a facility makes sure HACCP records are up to date, complete and accurate.
The following describes how to fill in each column.
Column 1. Process Step or Incoming Material – Enter in a description of each processing step or incoming material that has a CCP.
Column 2. CCP Hazard Number – The number given to each CCP is transferred into this column to make sure they correspond.
Column 3. Hazard Description– This column identifies the type of hazard that this CCP addresses.
Column 4. Critical Limits– This column identifies the standards that the product should be safely produced on. These standards must be clearly defined, objective and measurable.
Column 5. Monitoring Procedures – This column is broken down into four to identify monitoring procedures and how they will be used on the production floor. Monitoring procedures need to indicate:
- Who will perform the task (recorded in WHO column)
- What will be monitored (recorded in WHAT column);
- How it will be monitored (recorded in HOW column); and
- Frequency it will be monitored (recorded in FREQUENCY column).
Column 6. Deviation Procedures – This column is used to record deviation procedures and also to refer to documents that contain deviation procedure instructions.
Deviation procedures need to indicate:
- Who will perform the task
- What the task is
- How the task is to be performed
- Where this information will be recorded; and
- Cause of the deviation (if known).
Column 7. Verification Procedures – This column may be used to record verification or can refer to the documents that contain verification procedures. Verification procedures need to indicate:
- Who is responsible for the activity;
- What is being tested or examined;
- Why this is being tested or examined;
- How is the activity being carried out;
- When is the activity done (e.g. frequency); and
- Where the results or information are recorded.
Column 8. HACCP Records – This is a list of all documents and records connected with each CCP. State where each record can be found to assist facility employees.
Document and Record Control
A controlled document or record must contain the following:
- Title
- Creation/revision date
- Page number
- Prepared by/issued by
- Approved date
- Approval signature
By including this information on each page, a facility will be able to maintain control of the document or record. Include this information either in the header (top of the page), footer (bottom of the page) or in a combination of the two.
Controlled documentation also ensures that when the system is revised or updated, processors will use only the most up-to-date documents or records. This also helps processors make sure that changes are not made to the system without proper knowledge and approval.
Deviation Procedures and Corrective Actions
Although it may be easy to recognize when problems exist in a facility, it can be challenging to make sure that these situations are reported. It may also be challenging to make sure that corrective actions are taken and reported. Completing records of the corrective actions is important for the following reasons:
- Assists the company to prove due diligence
- Demonstrates a commitment to problem solving and the management of food safety issues
- May lead to improved employee performance
- Reduces unproductive, repetitive activities; and
- May reduce costs by revealing what activities aren’t working.
It’s important that corrective actions are reported in full each time there is a deviation or a change outside the acceptable limits in the food safety system. Complete documentation should include:
- The date and time the deviation was observed
- Nature of the deviation
- Whether product or food contact surfaces are affected
- What corrective actions are to be taken
- The timeframe for completion of corrective actions
- Signature of responsible employee; and
- Verification date, time and signature indicating that the activity was completed satisfactorily.
Documentation and Record Keeping
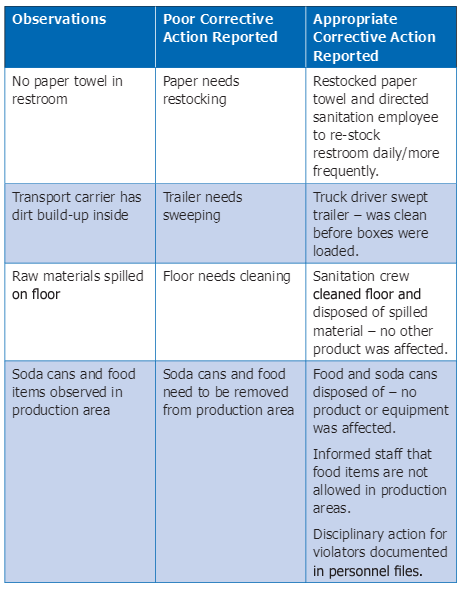
Below are some examples of poor and appropriate corrective action reports.
References
Lang T, Sambey, K., Fahner, K., Yaschuk, M., “2015 Food Safety Guidebook”, Chapter 3, Government of Alberta. Accessed at https://open.alberta.ca/dataset/da0b4f14-7249-427b-a17b-1688f21f82ce/resource/805dc063-e842-4049-b4f4-e66af8a7586e/download/2015-food-safety-guidebook.pdf, Sept 12, 2022.
