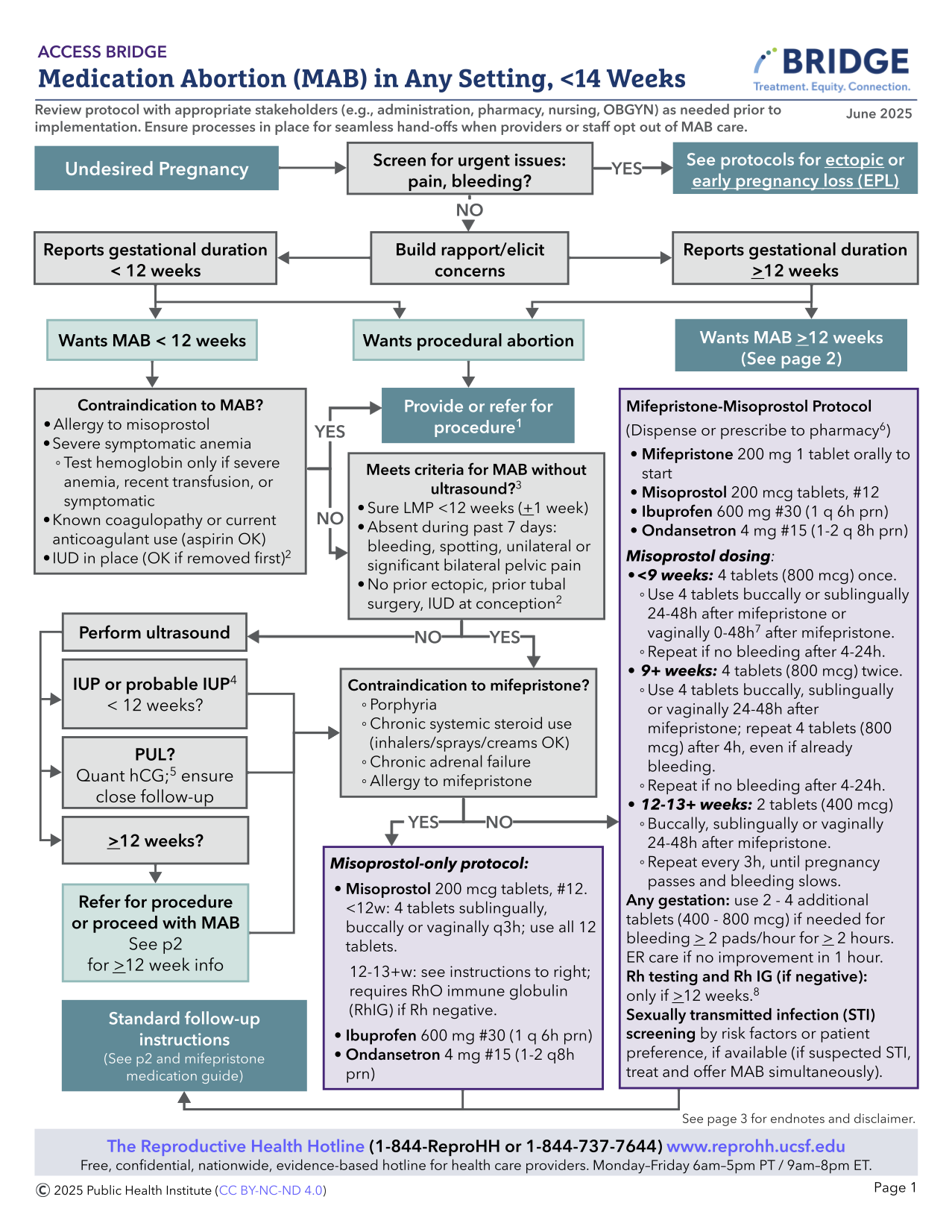
MIFEPRISTONE/MISOPROSTOL ABORTION
< 14 WEEKS: STEP BY STEP
Steps for telehealth are similar for in-clinic care. Telehealth visits can be done via synchronous or asynchronous modalities (using text- or email-based communication) or synchronous modalities (using audio only +/- video). Consider inequities in access to and comfort with electronic devices in addition to accessibility for people with disabilities (Datta 2022, Ko 2023, Koenig 2023). Consider checking state-based regulations and consulting legal resources regarding telehealth MAB here. For a more complete discussion of varying telehealth definitions and considerations, see Ch 10: Telehealth Medication Abortion).
MAB Appointment
Counseling
- Introduce yourself and build rapport
- Discuss confidentiality
- Discuss pregnancy and abortion options (medication vs. aspiration) and elicit any concerns
- Discuss abortion safety and reassure no interference with future pregnancy potential.
Eligibility
- Confirm pregnancy status – urine pregnancy test (UPT) ( self-report acceptable) or serum hCG, unless for use as period pills or advance provision (see section below)
- Pregnancy dating by last menstrual period (LMP) without ultrasound is appropriate with the following (see Ultrasound As Needed):
- Regular menses with sure LMP +/- 1 week of certainty
- If LMP is unknown, menses irregular, and/or person is using a hormonal contraceptive, a series of questions (Are you >10 weeks pregnant? Have you missed >2 periods? Are you >2 months pregnant?) may be used to determine eligibility for medication abortion (Ralph 2022).
- May use additional questions including certain timing of unprotected intercourse, recent negative UPT to support pregnancy dating
- No ectopic risk factors or symptoms (previous ectopic pregnancy, history of PID, IUD in place at conception or currently, prior tubal surgery, vaginal bleeding, significant unilateral or bilateral pelvic pain within the past week).
- If gestational duration is uncertain, ultrasound (US) is recommended to confirm gestational duration < 14 w on anticipated day of mifepristone use (see Ch 12 for MAB >14 weeks). Some clinicians use lower gestational limits to allow for dating error, the need for Rh testing and treatment, or other reasons.
- If one or more ectopic risk factors, recommend US if available, OR offer more extensive anticipatory guidance and education prior to MAB and ensure close follow-up (with serial hCGs or US if no bleeding as expected or persistent pregnancy symptoms).
- Special Circumstances:
- PUL (US with no definitive IUP identified): Early MAB can be done with appropriate counseling and follow up (see Ch. 3: PUL)
- Ectopic Pregnancy: If identified on US, or clinical picture is highly suggestive of ectopic, MAB should not be offered and ectopic pregnancy protocols should be followed. Whereas if US is only suspicious for ectopic, medications may be given while pursuing definitive diagnosis. Use shared decision making to determine medication timing (especially for those traveling long distances for care, who face barriers to returning for an MAB if ectopic is ruled out. Ectopic pregnancy rates are generally lower among people seeking abortion care (Edwards 1997).
- Review medical history for rare contraindications and precautions for MAB:
| Medication Abortion Contraindications and Precautions Adapted fromIpas 2022 |
||
| Medication Abortion | Mifepristone (can proceed with miso-only MAB) | |
| Contraindications |
|
|
| Precautions |
|
|
¹IUD self-removal is an option if there is no local access to affordable care.
²Recommend shared decision-making: MAB may be offered if procedural services are unavailable, misoprostol can be used with the person being within 30 minutes from an ED if the individual is fully informed of potential risks.
- No labs are required for MAB <12 weeks.
- The following should be considered:
- Rh testing: If 12+ weeks from LMP and Rh negative, discuss Rh immunoglobulin within 72 hours of MAB (ACOG 2024, NAF 2024, WHO 2022). (See Ch 5: Rh Isoimmunization). People can return to the clinic or access RhD Ig close to home. For people with unknown blood types and barriers to care, they could receive RhD Ig on the day of abortion empirically.
- Hemoglobin or hematocrit: If recent history of hgb <8, symptoms of anemia or history of blood transfusion. Rare clinically significant drop in hemoglobin after MAB.
- Chlamydia/gonorrhea screening: If symptoms, risk factors, or individual preference. MAB does not need to be delayed for suspected or confirmed STI (offer empiric treatment).
- Quantitative serum hCG: If trending to confirm abortion success or for PUL.
Informed Consent
- Confirm confidential contact information (or safe close contact if unavailable)
- Discuss safety and efficacy of MAB and review risks (see Complications Table)
- MAB is many fold safer than remaining pregnant, giving birth, and being postpartum in the U.S. (Stevenson 2023) although the magnitude of safety varies across global settings.
- Discuss high success rate, along with potential for needing additional misoprostol dosing or aspiration for unsuccessful MAB.
- Heavy or prolonged bleeding can occur in up to 3% of cases; management options include additional misoprostol, or non-urgent uterine aspiration. It is rare that emergent uterine aspiration or transfusion is indicated.
- Post-abortion endometritis is uncommon and occurs in <1% of cases. Atypical Clostridial infection is very rare (Fjerstad 2011, Meites 2010).
- There is no evidence-based regimen for mifepristone reversal. Not taking misoprostol after mifepristone may be associated with heavy bleeding leading to emergency care (Creinin 2020, Grossman 2015).
- Mifepristone is not a human teratogen (Turner 2024). Misoprostol is a teratogen in early pregnancy with slightly increased risk of congenital deformities from 1.8% to 4% (Auffret 2016, Vauzelle 2013) including Mӧbius syndrome – limb defects and facial (VII) and ocular (VI) nerve palsies.
- U.S. patients must review and sign a mifepristone patient agreement either in-clinic or via an electronic platform such as Docusign.
- Manufacturer’s Patient Agreement and Medication Guide: Danco (https://earlyabortionpill.com), or GenBioPro (https://genbiopro.com)
Counseling on Abortion Process
1. Review use of medications:
- Mifepristone:
- Blocks progesterone, the hormone that prepares the endometrium for implantation, resulting in endometrial disruption
- Avoid counseling that mifepristone will stop the pregnancy or stop cardiac activity (Crenin 2020)
- Mifepristone is an uncoated tablet which may be split in half or crushed if there is difficulty swallowing tablets (Blaszczyk 2023).
- Misoprostol:
- Stimulates uterus to contract and expel the pregnancy
- Administration route options:
- Vaginal: place tablets one finger length in the vagina. May sit or lay down for 30 minutes. Avoid the vaginal route if bleeding has already started. It may help with absorption to moisten pills prior to placement. Caution pill fragments may be visible on pelvic exam for days after use.
- Buccal: place tablets between gum and cheek for 30 minutes, ok if pills don’t dissolve; then swallow any remaining fragments.
- Sublingual: place tablets under tongue for 30 minutes, then swallow any remaining fragments. Sublingual misoprostol is very effective, but has a higher risk of gastrointestinal side effects.
- Dispensing 3 doses routinely (#12 200 mcg tablets) helps minimize in-person follow-up and increase management options, especially for those traveling significant distances and for those in states with restrictions or bans (Simmons-Duffin 2023).
- Due to increased sensitivity with gestation > 12-14 weeks, misoprostol dose is 400 mcg (vs 800 mcg for < 12 weeks).
- Patients can be instructed to hold doses in reserve in case of prolonged or excessive bleeding, or missed dose due to vomiting.
- If mifepristone is vomited less than 60 minutes after use, consider repeat dosing. If misoprostol is vomited (or falls out if used vaginally) less than 30 minutes after use, consider repeat dosing, or calling for further instruction.
- Pain Management:
- Pain may range from minimal, like a heavy period, to very significant. Many people report feeling poorly prepared for the amount of pain they experienced during a MAB (McCulloch 2024). It is important to consider ways to improve people’s experiences with MAB (Baraitser 2022, Friedlander 2022). See (Chapter 5, Pain Management) for further discussion of clinicians’ perception and bias in pain management.
- Risk factors for experiencing more pain with MAB include pre-existing dysmenorrhea or anxiety, emesis during abortion, insufficient pain medications, younger age, and increasing gestational duration (Kemppainen 2020).
- NSAIDs are evidence-based and the mainstay of pain management: ibuprofen 600-800 mg PO q6-8h or equivalent (Livshits 2009, Najmi 2023, Reynolds-Wright 2022). Recommend pre-medication 30-60 minutes before misoprostol use for best effect.
- Adjunct therapies
- Acetaminophen may be added, though evidence lacking.
- Tramadol (Dragoman 2021) and pregabalin (Friedlander 2018) may be helpful adjuncts, but not superior to NSAIDs and have additional side effects.
- Oxycodone is not superior to NSAIDs in pain scores or duration (Colwill 2019). Risk generally exceeds benefit in routine MAB. If given, 4-6 tablets is likely adequate.
- High-frequency TENS are effective for MAB pain (Goldman 2021).
- See Ch 5: Non-pharmacologic support for approaches including peer support, breathing, music, temperature, lighting and mindfulness.
- People routinely using cannabis for pain management may choose to use it during medication abortion, although evidence is lacking.
- Engage in person-centered care and shared decision-making to discuss the pros and cons of pain management options and offer what is feasible and reasonable.
- People with opioid use disorder in recovery may wish to avoid opioid prescriptions – though they are not contraindicated. Maximize adjunct therapies, and continue medication-assisted treatment as prescribed (Synder 2018).
- Antiemetics: may offer ondansetron, promethazine, metoclopramide, or over-the-counter dimenhydrinate (Dramamine), although the latter may be less effective. Consider using anti-emetics ahead of MAB medications if experiencing significant pregnancy nausea.
2. Provide anticipatory guidance for MAB process and expected symptoms:
- Ask if the patient prefers to have a support person available (virtual support is available at Reprocare and The Doula Project).
- Ensure they will have access to a bathroom after taking misoprostol.
- Approximately 10% – 20% of people experience nausea, vaginal bleeding and/or cramping after mifepristone alone and are still advised to take the misoprostol (De Nonno 2000) to optimize success.
- Common side effects of misoprostol include: nausea, vomiting, diarrhea, sore mouth/tongue, low-grade fever, chills and myalgias. These usually resolve within 24 hours of use.
- Cramping and pain occurs in >90% of patients, varies in intensity, on average peaks 4-6 hours after misoprostol.
- Vaginal bleeding is usually heaviest within 4-6 hours after misoprostol is taken, but can take 24 hours to start.
- A wide range of bleeding quantities are common, but bleeding is often heavier than a person’s normal menses and can include large clots. However bleeding may be lighter, especially with early abortions (<4-5 weeks). More research is needed to counsel people regarding expectations. At >9 weeks gestation, one may see a recognizable embryo or fetus. Visualization of pregnancy tissue is available at the MYA network and size and characteristics are available at Perinatology.com.
- Average bleeding duration is 13 days, but ranges 1-45 days (Davis 2000, Rodger 1989).
- Bleeding can stop and start intermittently until the next menses.
- A heavy first menses following MAB is common, typically 4-6 weeks later
- People can resume all normal activities (baths, tampons, sex, exercise) at any time after MAB with no increased risk of infection or complications
- Offer information about management of expelled pregnancy tissue:
- May choose to sit on a toilet while passing blood and pregnancy tissue and flush without reviewing the pregnancy tissue. People may choose a ceremony or ritual for the pregnancy tissue.
- Pregnancy tissue can be treated as other biological material (blood and pregnancy tissue can be flushed and soiled pads can be discarded, unless the individual wishes or local laws dictate otherwise). Information about pregnancy tissue >12 weeks can be found on Ineedana.com
- Offer to discuss contraception: remain aware that many prefer not to discuss at time of abortion (Brandi 2018). If interested, review options and timing for initiation, noting that the majority of methods can be initiated the same day mifepristone is taken (see Ch 7: Contraception). Reassure patients that abortion does not affect future fertility and that fertility may resume within a week of an abortion.
- For those who want to start a contraceptive method, it is recommended to start within 5 days of misoprostol so it is effective as soon as one decides to have sex again. If contraceptive start is delayed, suggest using a backup method for the first 7 days.
3. When to Call: Discuss how to reach clinician on call if:
- Any questions come up
- No bleeding 24 hours after last misoprostol dose (counsel to repeat misoprostol if no bleeding 6-24 hours after the last dose for highest efficacy).
- Soaked >2 maxi-pads for >2 consecutive hours (soaked front to back and side to side) or having dizziness or syncope
- Unmanageable pain despite taking analgesics
- Sustained fever >100.4° F or onset of fever >24 hours after misoprostol
- Abdominal pain, weakness, nausea, vomiting or diarrhea > 24 hrs after misoprostol
- Foul vaginal discharge associated with fever / illness. (Can reassure that abnormal smell is common and self-limited, if not associated with fever / illness).
- Pregnancy symptoms persist longer than 7-14 days
- A positive UPT 5 weeks after taking misoprostol (see Follow up section below)
- Rare indications to go to the Emergency Department (ED; see Managing Complications table below).
- Most concerns can be addressed with reassurance, anticipatory guidance, and remote management including instruction to take additional misoprostol doses or other medications as indicated.
- Excessive pain (with or without heavy bleeding) is often a sign of retained tissue/clots, and relief is usually achieved with NSAIDS and misoprostol 400 mcg (which may be sufficient with fewer side effects than 800 mcg).
- If an in-person visit is warranted, consider same- or next-day office visit instead of utilizing an ED.
- If an ED visit is needed, facilitation and/or appropriate counseling about political/legal concerns can help improve the person’s care experience, and reduce stigma or criminalization. If a person goes to ED, they can say “I’m pregnant and have been bleeding” as there are currently no urine or blood tests to detect mifepristone or misoprostol.
- If denied emergency care or having other legal concerns in the U.S., free legal advice is available at the Repro Legal Helpline.
- Most concerns can be addressed with reassurance, anticipatory guidance, and remote management including instruction to take additional misoprostol doses or other medications as indicated.
Additional Educational Resources:
- How To Use Abortion Pill: https://www.howtouseabortionpill.org/
- Safe2Choose: https://safe2choose.org/abortion-information/resources/
- RHAP How to Use Abortion Pills: https://www.reproductiveaccess.org/resource/mabfactsheet/
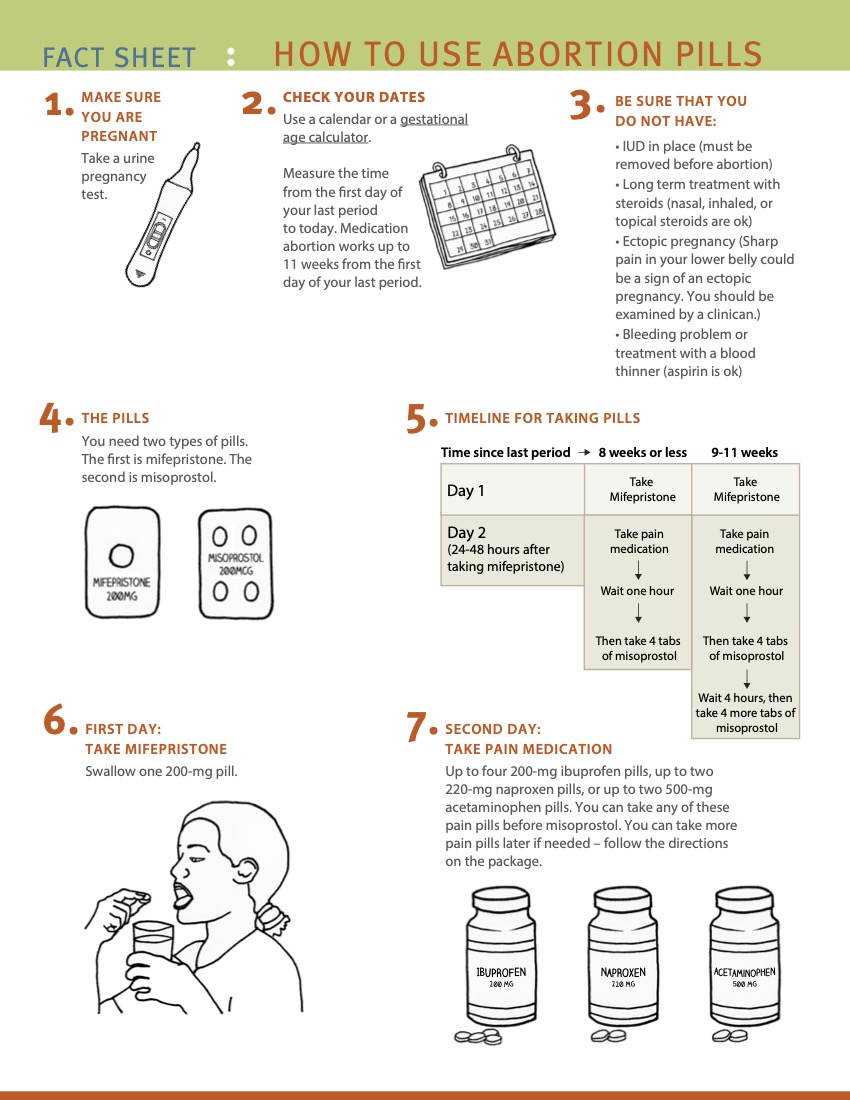 |
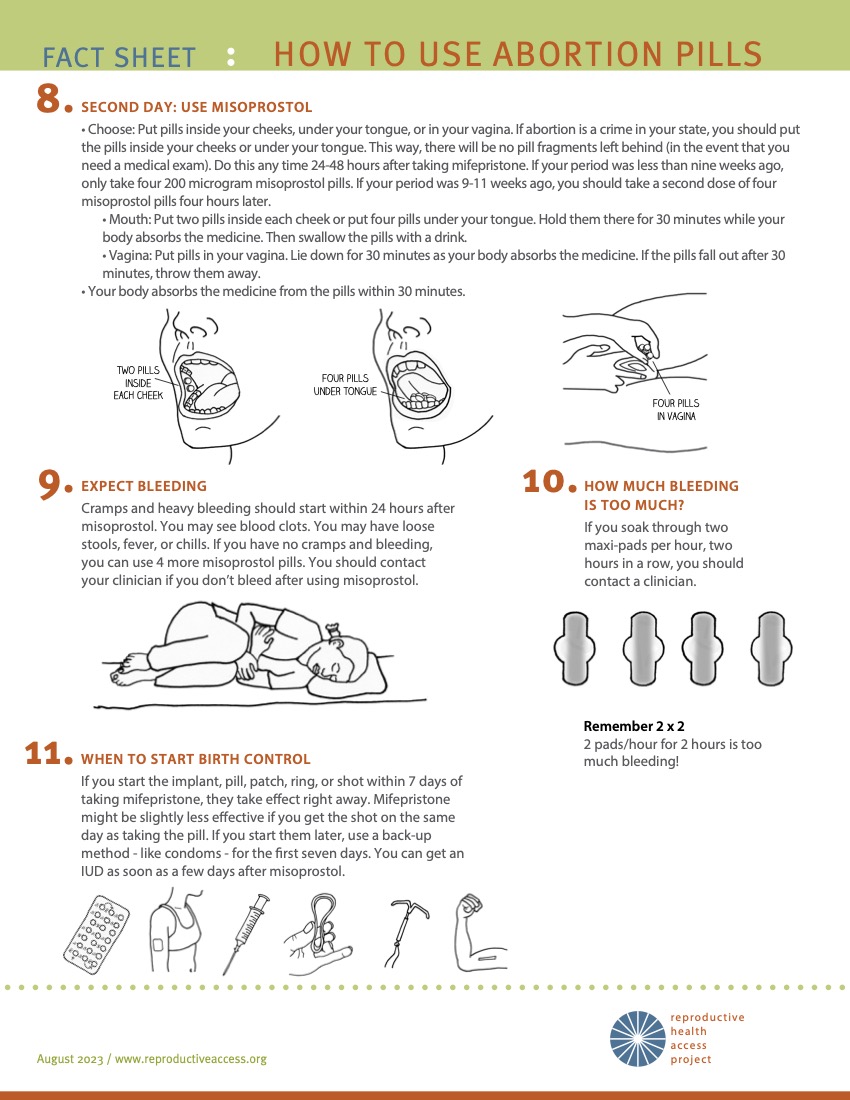 |
(Source: Reproductive Health Access Project)
Dispensing Mifepristone
- The US Food and Drug Administration (FDA) has placed a Risk Evaluation and Mitigation Strategy (REMS) for Mifepristone. This requires healthcare providers or pharmacies to register with a distributor to prescribe or directly dispense the medication. Only one clinician in a clinic or pharmacy needs to be registered in order for their colleagues to also directly dispense Mifepristone.
- Prescribers certifying must complete a prescriber agreement with either with Danco or GenBioPro.
- The medications can be dispensed in the office (and can be taken at home without direct supervision when taking them unless local laws dictate otherwise), can be mailed via a medication delivery system, or be prescribed to a certified local or mail order delivery pharmacy.
- Please see Access Delivered Provider Toolkit for more information on setting up mail-order pharmacy distribution.
Follow-up Telehealth or Office Visit – Day 7 – 14
Follow up with a clinician after MAB is optional. Many telehealth MAB models use a brief questionnaire completed via text or email. Clinics, insurers or state laws may have specific requirements for follow up.
- Review the person’s course since taking medications, including timing and extent of bleeding and cramping, and pregnancy symptom resolution.
- Symptoms that merit additional misoprostol or a follow up call or visit:
- No bleeding (see When To Call above)
- Does not feel that the pregnancy has passed or reports still “feeling pregnant”
- A persistently positive UPT after 5 weeks. Persistent positive UPT 8% at 5 wk vs. 20% at 4 weeks after MAB (Raymond 2021), thus testing at 5 weeks minimizes unnecessary intervention.
- Continued or worsening symptoms of pregnancy (nausea, breast tenderness)
- Repeat miso or mife/miso can be tried before an in-person visit if preferred
- Symptoms that require in-person evaluation:
- Continued heavy bleeding without improvement, unresponsive to additional misoprostol
- Significant pain unrelieved by analgesics or additional misoprostol
- Fever above 100.4 greater than 24 hours after misoprostol
- Symptoms of hypovolemia (ex. dizziness or syncope)
***Repeat miso or mife/miso can be tried before an in-person visit if preferred
- Success of abortion can be assessed by a) clinical history in conjunction with home UPT at 5 weeks, b) by serial quantitative hCG testing, or c) by US (NAF 2024).
- Clinical history (assessing symptoms by telehealth or phone) is acceptable (Clark 2010), when paired with home UPT at 5 weeks (Schmidt-Hansen 2020b, Grossman 2011, Oppegaard 2015). May dispense additional UPT.
- When serial hCG protocol is used, a decrease from baseline hCG of 50% by 72 hours, 60% by 4-5 days (Pocious 2016), and 80% by 7 days from initiating treatment (Fiala 2003) is consistent with a successful MAB.
- As hCG physiologically declines in the later first trimester, assess symptoms in conjunction with hCG if clinically suspect ongoing pregnancy.
- When US is used, success is determined by demonstrating the absence of a previously identified pregnancy (gestational sac or embryo).
- Asymptomatic heterogenous intrauterine material or thickened endometrium is common after MAB and does not imply actual retained products of conception. Intervention should not be offered if no pain nor significant or bothersome bleeding, regardless of US findings (see US as Needed: Successful Abortion below)
- Review interpretation of clinical course and any follow up results. Have the person contact the clinic for late-onset heavy bleeding or concerns warranting evaluation.
- Offer to discuss contraception, if desired (See Ch. 7: Contraception)
| Proposed Criteria for Aspiration after MAB |
|
Emergent
Non-emergent
|
Sample MAB Protocol
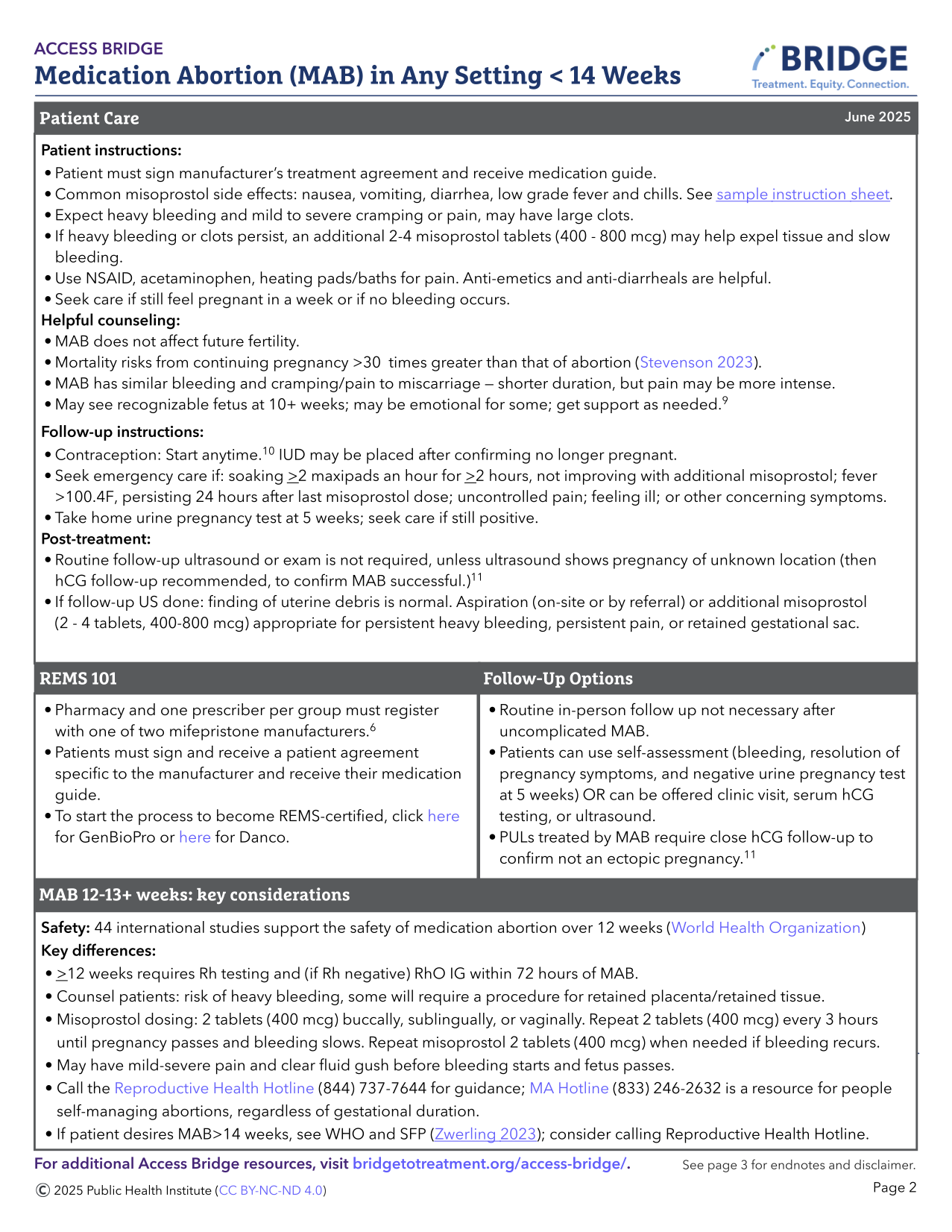 |
 |
To view or download, visit (Access Bridge).