3
Nazanin Beigi, Erin Bryan, Hannah Dobson, and Niralya Sundararajan
Abstract
When one is told to imagine a frog, they would likely think of a species that is the size of their palm. However, the species this chapter will be dedicated to is not nearly as large, but make no mistake—it is certainly a persistent little creature. Bromeliohyla dendroscarta, also known as the greater bromeliad Tree Frog, is part of the Hylidae family. Located at high altitudes within the cloud forests of Mexico, B. dendroscarta nests in the phytotelmata, or water-filled cavities, of bromeliad plants. A combination of its secluded habitat and declining population rates have left B. dendroscarta mostly unknown to the world; in fact, it had been classified as extinct from 1972 to 2017, a staggering 45 years. This chapter will discuss the characteristics and traits of B. dendroscarta, alongside factors for its decline and conservation strategies.

Figure 1: Adult Bromeliohyla dendroscarta, by Victor Vásquez-Cruz in Vásquez-Cruz et al. (2019), BY-NC-ND
Introduction
Legend has it, when the vast sea of fog in the cloud forest parts and the rays of the sun shine down upon the mossy earth, witnessing the golden shine of the greater bromeliad tree frog is an experience surpassed by none other. Prowling through the fields of moss and the labyrinths of trees in Central Mexico, researchers have been on the hunt for the frog since its last sighting in 1972. As stories and tales are routinely passed down about this fabled creature, the line between fact and fiction has been blurred. However, through all these stories carved on tablets, passed from word of mouth, or written in novels, one identifying factor remains consistent—a small, golden head poking out of a blooming bromeliad affixed to a tree.
Physical Traits
This species is, in fact, endemic to Mexico (Bañuelos et al. 2017). The greater bromeliad tree frog (Bromeliohyla dendroscarta) is a small frog—only a 30-millimeter snout to vent length (which measures from the nose to the cloacal slit) for males and a 36-millimeter length for females. One eye is equal in length to the snout, and the toes are webbed and about one and two-thirds of a millimeter. The adults have a variety of yellow and white tones across their bodies and gold irises (Vásquez-Cruz et al. 2019). Most of B. dendroscarta’s skin is generally smooth but the ventral surfaces of the stomach, throat, and hind limbs are granular. Although most physical traits across relative species are similar, other species in the Hylidae family include terrestrial and semi-aquatic tree frogs.
Figure 2: Side and top view of Bromeliohyla dendroscarta, photos by Alfonso Aceves-Aparicio (A, B) and José Luis Aguilar López in Bañuelos et al. (2017), CC BY-ND 4.0
Habitat
Bromeliohyla dendroscarta are generally located in the cloud forests that are found in the highlands of Central Veracruz, Mexico. The cloud forest is one of the most biodiverse vegetative assemblages. However, due to this, it is highly threatened by deforestation. (Peralta-Hernández et al. 2020). Knowing more about general amphibian species richness in cloud forests may help to understand the general context of B. dendroscarta population trends (Hernández-Salinas and Ramírez-Bautista 2012). Of course, as important as the habitat is to these frogs, it’s what’s in the habitat that is crucial.
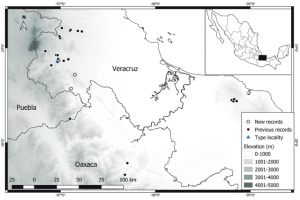
Figure 3: Bromeliohyla dendroscarta range map. “Geographic distribution of Bromeliohyla dendroscarta, showing the new localities” in Vásquez-Cruz et al. (2019), BY-NC-ND
Micro-Frog in a Micro-Habitat
The habitat that current bromeliad tree frogs live in presents a mutually beneficial relationship between themselves and the bromeliad plant. Generally, bromeliad tree frogs inhabit damp cloud forests. Since frogs are reliant on plants and trees (both for development and nutrition), they have a very close relationship with bromeliad plants within their habitat. Some bromeliad plants are extremely colorful and have lots of branches, while others are a solid green color. The structure of the plant is formed in such a way that it is able to capture water, almost like a pool—that is where the famous name, the bromeliad pool, is derived from. Insects also flock to these pools supplying the tree frogs with ample nutrition. This micro-habitat and its amenities have led to an incredibly strong relationship between bromeliad tree frogs and the bromeliad plant.
Relationship With Bromeliad Plant
Many factors of the bromeliad plant contribute to the survival of Bromeliohyla dendroscarta. The big leaves by themselves provide shade for the frog as well as protection from predators by camouflage. Another benefit of the leaves is for the eggs. At times, the frogs will lay their eggs into a water-filled cavity within the plant, known as the phytotelma, to ensure the eggs have access to water (a semi-aquatic style). Based on the greater Hylidae family, some frogs will use ponds or go into trees to lay their eggs. Upon hatching, the tadpoles have an average total length of about 7 mm. Once hatched, the species have suckers that allow them to latch themselves onto rocks, an important adaptational skill that helps with protection and survival. In addition, the microbiomes the leaves of the bromeliad plants create for the bromeliad tree frogs benefit the frogs early in their life. The population of this tree frog is intrinsically linked to the population of the bromeliad plant as it is the plant that makes up the frog’s habitat for much of its life (Stuckert et al. 2009). The tadpoles of the bromeliad tree frog eat a variety of things found in bromeliad plants, such as detritus, and the adult frogs mostly consume insects and other invertebrates.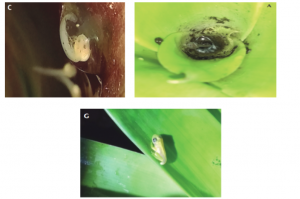
Figure 4: Top left) B. dendroscarta embryo. Top right) B. dendroscarta tadpole feeding off detritus. Bottom center) B. dendroscarta juvenile. Photos by Arleth Reynoso-Martínez (top left), Arleth Reynoso-Martínez (top right), Víctor Vásquez-Cruz (bottom) in Vásquez-Cruz et al. (2019), BY-NC-ND
As important as the bromeliad plant is for the tree frogs, the tree frogs are just as beneficial for the plants. Bromeliad trees and frogs that dwell within present an example of a mutualistic symbiotic relationship. Together, the bromeliad plant and the greater bromeliad tree frog aid in nutrient cycling, provisioning food and water, and maintaining community structure and biodiversity (Ladino et al. 2019). These plants form a virtual aquarium holding up to 20 liters of water which attract insects, spiders, mites, etc, that the tree frogs feed on. These tree frogs that live in bromeliads bring nutrients to the plant in their droppings. The consumption of these insects in their larval and mature forms helps to keep vectors for disease to a minimum. Litter decomposition in tanks aids in the redistribution of nutrients at the same time as the feces of the frog adds nitrogen to the plant (Ladino et al. 2019). Tree frogs also play an important aspect in the local food web as they are prey to larger predators such as snakes.
Figure 5: Distinct photos of bromeliad plants of the genus Tillandsia. Photos by Alfonso Kelly-Hernández (left) and Raul Andrés Díaz-Ramos (right) in Vásquez-Cruz et al. (2019), BY-NC-ND
Life History
Researchers holding a study between 2015 and 2019 in Mexico heard the call of an unidentified tree frog up within the canopy (IUCN 2019). Upon further investigation and location of a specimen, it was made clear that this frog was not a member of one of the local, more well-known species but rather a member of the long thought extinct greater bromeliad tree frog (IUCN 2019). They have been found in single populations across areas in Belize, Guatemala, Honduras, and regions of Brazil. Our focus will be on Mexico. Spending its life in the upper canopy of the cloud forests, it is incredibly hard to get an accurate account of the frog’s population and thus local ecologists had thought it had died out in 1974. With this discovery, some very interesting things can be deduced about the relationship between amphibian population and humans’ impact on the environment.
Fractured by Humanity (Population Trends)
Demographic characteristics of the Mexican tree frog, Hylidae family, fluctuate due to the ripple effect of changes in environmental factors, precipitation, and temperature. (Cruz-Ruiz et al. 2015). This contributes to changes in the amphibian population and conservation status. These small frog species are at a high disadvantage due to habitat fragmentation. The study indicated a 2:1 sex ratio for male:female. In the Central Mexican Plateau, 44.7% of the area is used for agriculture. This presents the possibility of a reduction in the suitability of existing habitat patches which may affect pond-breeding amphibian population dynamics. Even though it is not the bromeliad tree frog, it is the same family located in the same exact habitat (Central Mexico). Simultaneously, the miscategorization of geologically and visually close tree frog species also heavily contributes to the preserved population size. In the past ecologists have believed that the Hyla cembra had gone extinct, but further research had shown that living specimens had been misclassified as Hyla mykter (Mendelson and Canseco-Márquez 2002).
While other research has found B. dendroscarta to be present as of 2017 in Veracruz, B. dendroscarta are reported as still missing from the Sierra Juarez as of 1972. Callings of unidentifiable frogs were heard from bromeliads in trees near Oaxaca and Veracruz (Delia et al. 2013). This is additional confirmation of B. dendroscarta being traditionally located in the highland region of Veracruz.
In addition, there was noted to be a link between altitudinal habitation and risk level in amphibian species, with many critically endangered species being located at higher elevation levels. This is due to the low dispersal rate and high fidelity towards breeding sites, which are reducing in number due to deforestation. B. dendroscarta was found to be located at an elevational range of about 1450 km, with a max elevation of 1900 km (Caviedes-Solis et al. 2020).
Due to limited research findings, it is important to understand the history of amphibians and their trend of decline/extinction throughout the world (Stuart et al. 2004). In the Hylidae family, enigmatic decline and reduced-habitat are leading to amphibian declines and extinctions (Stuart et al. 2004). This is illustrated as tank bromeliad frog species and arboreal frog species were absent from second-growth forests, forests that had previously slashed and burned to make space for agriculture (Galindo-Leal et al. 2003).
Over 64% of amphibians are suffering some sort of population decrease in Mexico, in contrast to only about 1.1% of amphibian populations increasing, with B. dendroscarta included in the former. It was also documented that the IUCN Red List had registered several species of threatened frogs as being non-native to Mexico when further studies confirmed that they were indeed endemic, such as Ambystoma mavortium and Craugastor galacticorhinus (Frías-Alvarez et. al. 2010). The potential risks of losing species entirely through extinction were documented in terms of the phylogenetic information lost. B. dendroscarta was noted to have a fairly high level of evolutionary distinctiveness (Caviedes-Solis et al. 2020).
Ecological and Societal Impacts
A majority of frog species today speciated from tropical frogs, and Bromeliohyla dendroscarta is no exception. The tropical conservatism hypothesis explains that groups with high species diversity originated in the tropics and speciated, spreading from there. B. dendroscarta is the result of years of evolution and has unique phylogenetic information. In addition, Middle America contains the second-highest root age (the age where Hylids emerged within the area) of any Hylid habitat at 60.65 million years, so it presents the most time for species to specialize themselves into niches (Wiens et al. 2006).
Though Bromeliohyla dendroscarta is one of many endemic species in Mexico that occupy two physiographic regions and are highly vulnerable, each individual species of endemic amphibian plays a significant role in their ecosystem (Johnson et al. 2017). Endemic herpetofauna make up 61.1% of the herpetofauna in Mexico (Johnson et al. 2017). B. dendroscarta is a part of the majority of the amphibians in the ecosystems of the country, making it a member of a group with considerable ecological impact. The greater bromeliad tree frog is part of understanding the effects that the complex changes to the hydrosphere, atmosphere, and lithosphere are having on Earth’s biodiversity.
Developing Conservation Strategies
The greater bromeliad tree frog and its survival rate have been changing due to the modified landscapes (Bañuelos et al. 2017). Reports showed how deforestation and disturbance of the cloud forest are major threats to its persistence. Knowing how dependent the tree frogs are on bromeliad plants for survival emphasizes exactly why deforestation causes a sharp decline in population size. A decline in habitat to lay eggs, to provide nutrients to the tadpoles for development, to protect themselves against predators—all are reflected in their continuous extinction. Because the tree frogs have not been found in suitable habitats since 1974, species like the chytrid fungus, Batrachochytrium dendrobatidis, are a serious threat to its survival. The chytrid fungus is a fungus that causes the disease chytridiomycosis in amphibians. This disease is deadly and has caused a sharp decline toward extinction for many species, including the bromeliad tree frog. Based on several sampling methods, reports have shown that the habitats they were in years ago, compared to the habitats they occupy now, have seen an extreme decline in both frog size and population size.
Amphibian populations in Mesoamerica are in sharp decline due to human economic factors (Wilson et al. 2013). The usages of slash and burn forests as a profitable cultural and economic staple of local agriculture leads to intensive loss of habitat (Galindo-Leal et al. 2003). The rehabilitation of this land usage also does little to help as second-growth forests are also not proper habitats (Galindo-Leal et al. 2003). Second-growth forests are inhospitable for bromeliad tree frogs because the main staple of their habitat the bromeliad plants can only survive on old-growth trees. Wilson et al. (2013) make it clear that in order to reverse the damage human and market factors have had on these species’ population, steps must be taken to understand the root of the issue, continue to document the issue, fix the immediate issue, and create policies to stop this from happening again.
Another reason that Bromeliohyla dendroscarta may be especially vulnerable is that it is one of the Hylids that lives in smaller, more isolated subpopulations that have been made small and isolated by deforestation and other modes of their habitat being fragmented (Urbina-Cardona and Loyola 2008). One of the suggestions for mitigating the negative ecological impact of the endangerment of these amphibians is to create conservation area networks to better coordinate the protection of species like B. dendroscarta. These conservation area networks will restore connectivity through the efforts of conservation groups being coordinated in a network, as well as eventually creating corridors between fragmented populations (Urbina-Cardona and Loyola 2008). In addition to developing these coordinated networks, restoring connectivity between fragmented populations is another recommended conservation strategy (Urbina-Cardona and Loyola 2008). Since there are seemingly no conservation effort precedents for B. dendroscarta due to its recent rediscovery, all conservation recommendations made are based on those of endemic amphibians at large (Loucks et al. 2008). This is one of the unique challenges of addressing the conservation of a species thought to be extinct until 2017.
Conclusion
And thus, the tale of the elusive little critter, the greater bromeliad tree frog, lives on. A history of destruction, disappearance, and the return of a monarch… such a quaint species should persist for future generations, don’t you think?
References
Britannica, Editors of Encyclopaedia Britannica. 2001 . Life in a bromeliad pool [Internet]. [cited 2021 Mar 17]. Encyclopeaedia Britannica. Available from: https://www.britannica.com/topic/Life-in-a-Bromeliad-Pool-1688529.
Bañuelos PG, López JL, Pineda E, Garcia-Vinalay A 2017. Rediscovery of Bromeliohyla dendroscarta at the type locality: a threatened treefrog surviving in a highly human modified landscape in Mexico. Mesoamerican Herpetology [Internet]. [Cited 2021 Feb 23] 4: 684-688. http://mesoamericanherpetology.com/uploads/3/4/7/9/34798824/othercontributionnotes_sept2017.pdf
Caviedes-Solis IW, Kim N, Leaché AD. 2020. Species IUCN threat status level increases with elevation: A phylogenetic approach for Neotropical tree frog conservation. Biodiversity and Conservation 29(8): 2515–2537. https://doi.org/10.1007/s10531-020-01986-8
Cruz-Ruiz G, Venegas-Barrera CS, Sanchez-Sanchez H, Manjarrez J. 2015. Temporal stability of an endemic Mexican treefrog. PeerJ 3: e1274–e1274. https://doi.org/10.7717/peerj.1274
Delia JRJ, Whitney JL, Burkhardt T. 2013. Rediscovery of ‘lost’ treefrogs from the Oaxacan highlands of Mexico. Biodiversity and Conservation 22: 1405–1414. https://doi.org/10.1007/s10531-013-0481-9
Frías-Alvarez P, Zúñiga-Vega JJ, Flores-Villela O. 2010. A general assessment of the conservation status and decline trends of Mexican amphibians. Biodiversity and Conservation 19(13): 3699–3742. https://doi.org/10.1007/s10531-010-9923-9
Galindo-Leal C, Cendo-Vazquez JR, Calderon R, Augustine J. 2003. Arboreal frogs, tank bromeliads and disturbed seasonal tropical forest. Contemporary Herpetology (1): 1-12. https://doi.org/10.17161/ch.vi1.11966
Hernández-Salinas U, Ramírez-Bautista A. 2012. Diversity of amphibian communities in four vegetation types of Hidalgo State, Mexico. The Open Conservation Biology Journal 6(1): 1–11. https://doi.org/10.2174/1874839201206010001
IUCN SSC Amphibian Specialist Group. 2019. The IUCN Red List of Threatened Species [Internet]. International Union for Conservation of Nature and Natural Resources. [Updated 2019 Oct 21; Cited 2021 Feb 23]. https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T55466A53954731.en
Johnson JD, Wilson LD, Mata-Silva V, García-Padilla E, DeSantis DL. 2017. The endemic herpetofauna of Mexico: organisms of global significance in severe peril. Mesoamerican Herpetology 4(3): 544–620. http://mesoamericanherpetology.com/uploads/3/4/7/9/34798824/mh_4-3_johnson_et_al.pdf
Ladino G, Ospina-Bautista F, Estévez Varón J, Jerabkova L, Kratina P. 2019. Ecosystem services provided by bromeliad plants: A systematic review. Ecology and Evolution 9(12): 7360–7372. https://doi.org/10.1002/ece3.5296
Loucks C, Ricketts TH, Naidoo R, Lamoreux J, Hoeckstra J.. 2008. Explaining the global pattern of protected area coverage: relative importance of vertebrate biodiversity, human activities and agricultural suitability. Journal of Biogeography 35(8): 1337-1348. https://doi.org/10.1111/j.1365-2699.2008.01899.x
Mendelson III JR, Canseco-Marquez L 2002. Rediscovery of the rare treefrog, Hyla cembra Caldwell, in Oaxaca, Mexico. The Southwestern Naturalist 47(3): 459–461. https://doi.org/10.2307/3672505
Peralta-Hernández R, Peralta-Hernández RO, Parra-Olea G, López-Velázquez A. 2020. Amphibians and reptiles from cloud forest at Cumbre de Tonalixco in the central Veracruz highlands of Mexico. Reptiles & Amphibians 27(3): 501-505. https://doi.org/10.17161/randa.v27i3.14898
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306(5702), 1783–1786. https://doi.org/10.1126/science.1103538
Stuckert AMM, Stone JP, Asper JR, Rinker MG, Rutt CL, Trimmer NC, Linquist ED. 2009. Microhabitat use and spatial distribution in Picado’s Bromeliad Treefrog, Isthmohyla picadoi (Anura, Hylidae). Phyllomedusa: Journal of Herpetology 8(2): 125-134. https://doi.org/10.11606/issn.2316-9079.v8i2p125-134
Urbina-Cardona JN, Loyola RD. 2008. Applying niche-based models to predict endangered-hylid potential distributions: Are Neotropical protected areas effective enough? Tropical Conservation Science 1(4): 417–445. https://doi.org/10.1177/194008290800100408
Vásquez-Cruz V, Canseco-Márquez L, Reynoso-Martínez A. 2019. Distributional and natural history notes for Bromeliohyla dendroscarta (Anura:Hylidae) in Veracruz, Mexico. Phyllomedusa 18(1): 27-36. http://dx.doi.org/10.11606/issn.2316-9079.v18i1p27-36
Wiens JJ, Graham CH, Moen DS, Smith SA, Reeder T. W. 2006. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: Treefrog trees unearth the roots of high tropical diversity. The American Naturalist 168(5): 579-596. https://doi.org/10.1086/507882
Wilson LD, Johnson JD, Mata-Silva V. 2013. A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphibian and Reptile Conservation 7(1): 97–127. http://amphibian-reptile-conservation.org/pdfs/Volume/Vol_7_no_1/Special_Mexico_Issue_ARC_7_1_1-47_e61_high_res.pdf
(Singular form: phytotelma) a small water-filled cavity in a terrestrial plant, which may serve as the habitat for associated fauna and flora.
referring to the front or lower side of an organism
a collection or gathering of things or people.
the number of different species represented in a certain ecological community or region
the microorganisms in a particular environment (including the body or a part of the body)
A close and long term relationship between two different biological species. Includes mutualistic(both species benefit), commensalistic(one organism benefits while the other is unaffected), and parasitic(one organism benefits while the other is harmed).
The variety of life in the world or in a particular habitat or ecosystem.
a system of interlocking and interdependent food chains
the process during which a large expanse of habitat is transformed into a number of smaller patches of smaller total area isolated from each other by a matrix of habitats unlike the original (Fahrig, 2003)
relating to height above sea level or ground level.
A method of igniting a forest or brush until it has burnt to the point of complete ash
relating to the evolutionary development and diversification of a species or group of organisms
A geographic region with characteristic geologic features, and often specific subsurface rock type or structural elements.
the reptiles and amphibians of a particular region, habitat, or geological period.
The action of clearing a wide area of trees.