28
Learning Objectives
Knowledge
- Recognize the indications for the use of contrast media in the study of various organs/organ systems using fluoroscopy, CT, DSA, and MRI
- Know the risks and side effects of commonly used contrast media.
- Determine the use of the various timing phases of contrast media application and their respective values according to the clinical problem.
- Define nephrogenic systemic fibrosis (NSF), related to MRI contrast, and the measures that can be taken to minimize this risk
Competancies
- Recognize if a CT is contrast-enhanced, or not
- Recognize various types of contrast-enhanced fluoroscopic examinations
- Communicate the risks and benefits of contrast media application for various common radiological examinations (including radiography, fluoroscopy, DSA, CT, and MRI) to patients and their families
A fundamental factor that results in diagnostic images being clinically useful is the concept that different anatomic tissues have differential appearances on imaging, whether it be density, intensity, or echogenicity. For example, the density of the liver on Computed Tomography (CT) does not match that of the gallbladder, and therefore, these structures can be differentiated on CT images.
When tissues are very similar in appearance to the structures around them, e.g. arteries vs. veins in the hilum of the lung, it is sometimes necessary to utilize a contrast agent to help differentiate one structure from another. The administered contrast alters the way that x-rays pass through the patient to reach the image detector, alter the response of tissues to ultrasound or magnetic fields, and hence effect the appearance of the resulting images making it different from an image without contrast agent administered. Malignant cells often display differential contrast enhancement than normal tissue making them more visible on imaging. Often it is important to time image acquisition based upon the length of time from contrast injection to capturing the image to accentuate the visibility of arteries, veins, and organs, optimizing the detection of abnormalities.
There are a number of different forms of imaging contrast (gas, liquid, suspension) allowing for delivery by mouth, per rectum, intra-luminal, or intravenous/intra-arterial routes. Each different delivery mode has unique applications, for example, oral contrast, a suspension of barium, is used for fluoroscopy (esophagrams, upper gastrointestinal series, and small bowel follow-throughs). Particulate suspensions of barium can only be administered into the intestinal tract. Administration, or leakage, of barium outside the intestinal tract may have severe adverse outcomes.
The majority of contrast used in x-ray based imaging is water soluble. This is a pharmacologic agent bound to molecules of iodine and it is administered intravenously, intra-cavitary, or intra-arterially. The presence of the iodine prevents the x-rays from penetrating the contrast enhanced tissue, resulting in these structures appearing denser (more opaque) on imaging. For example, dense contrast in veins or arteries can be almost as dense as bone on CT imaging. Knowledge of the physiology of blood-flow and the anatomic distribution of arteries and veins is also important in the interpretation of contrast enhanced images.
Because Magnetic Resonance Imaging (MR) imaging does not use x-rays, contrast enhancement for MR is a paramagnetic, gadolinium based, contrast agent (GBCA) that can be injected intravenously or intra-articularly. The use of paramagnetic contrast agents for MR functions to alter the signal from the tissues in which it concentrates, which is accomplished by altering the response of water molecules in the tissues to the magnetic field and radiowaves that are used in MRI.
Ultrasonography with contrast is performed infrequently. Ultrasound contrast is gas containing micro-bubbles, or microspheres, for injection. The gas encapsulated within the shell of the microsphere results in increased echogenicity of the tissues that harbour the contrast agent. When injected into the blood stream, ultrasonographers can visualize the echogenic bubbles or spheres.
Contrast Agents in Imaging:
- Many forms of contrast (gas, liquid, suspension) with many forms of delivery (by mouth, per rectum, intra-luminal, intravenous, and intra-arterial)
- CT most often uses water-soluble contrast; iodine bound to pharmacologic agents
- MR uses gadolinium based contrast agents (GBCA)
- Ultrasound can use gas containing micro-bubbles or micro-spheres as contrast agents
Modality Specific Contrast Utilization
Fluoroscopy
Fluoroscopy is an x-ray based modality used for a wide variety of imaging procedures. Examples of some of the procedures include, barium swallows, barium enemas, interventional procedures (catheter insertion, angioplasty, and angiography), myelography, upper gastrointestinal series, and small bowel follow-through and arthrography. For double contrast barium enemas both gas and barium are introduced into the colon to acquire optimal images of the mucosa of the bowel. Therefore, fluoroscopy can potentially employ all three forms of imaging contrast (water soluble, suspension, and gas) for a wide variety of procedures.
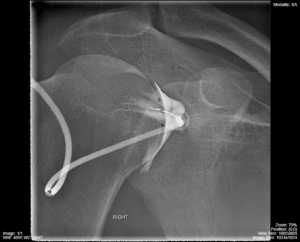
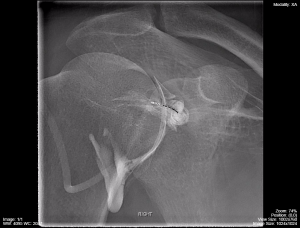
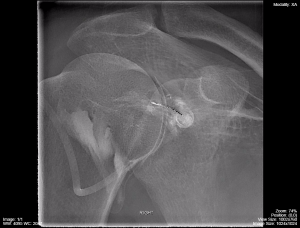
Figure 4.1 demonstrates a shoulder arthrogram where water soluble contrast has been injected into the joint space of the shoulder as part of a diagnostic or therapeutic procedure. The needle was maneuvered into the joint space using fluorocopy and the dispersal of the injected contrast is monitored with sequential images.
Computed Tomography
There are a wide variety of indications for contrast in CT. CT has excellent contrast resolution and this feature can be used to detect subtle differences in tissue enhancement and help to detect liver metastases, central tumor necrosis, and other pathologies that rely upon the differential concentration of injected contrast agent. On a daily basis CT uses the largest volume of water soluble contrast agent in the entire Diagnostic Radiology department. Examples of the use of contrast for CT imaging include, arterial phase imaging, CT Angiography (CTA) such as pulmonary arterial imaging for a Pulmonary Embolus (PE) study, and lung cancer staging to differentiate between lymph nodes, veins, and arteries in the pulmonary hilum. Oral, water soluble, contrast can also be used in the intestinal tract to facilitate visualizing the stomach, small bowel, or large bowel. For CT, bowel contrast must be water soluble as barium creates very prominent artifacts due to the atomic number of barium absorbing a large amount of the administered x-rays. Gas, in the formed of injected carbon dioxide, is used for the performance of CT Colonography.
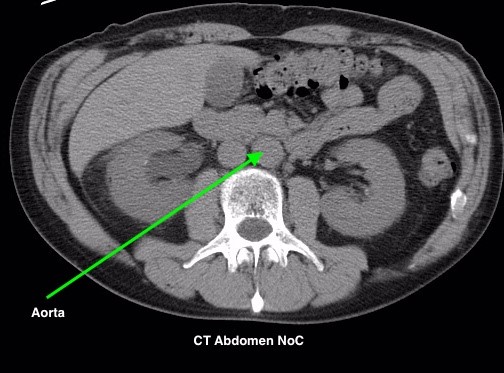
Figure 4.2 depicts two sets of CT scans, one set is without contrast (denoted as No C), and one set is with intravenous contrast (denoted as C+). The images are scans of the abdomen, with arrows pointed to the abdominal aorta and the common iliac arteries. The study with contrast in the arteries makes it easier to differentiate the arteries from the surrounding tissues, especially downstream of the aortic bifurcation. The images were acquired quite early after the intravenous injection of the contrast agent in the arterial phase of enhancement, contrast in the superior mesenteric artery, the renal arteries, and the kidneys is also noted.
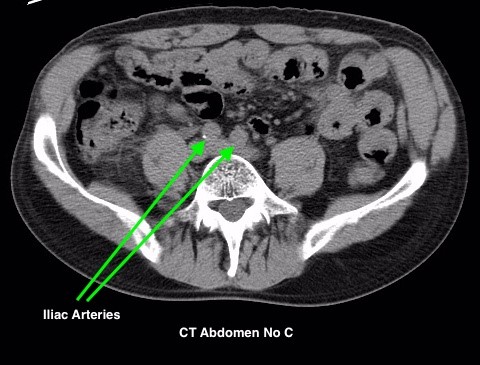
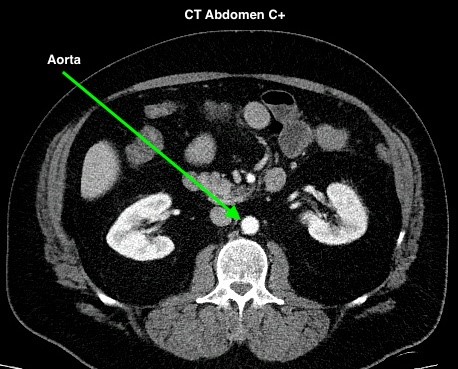
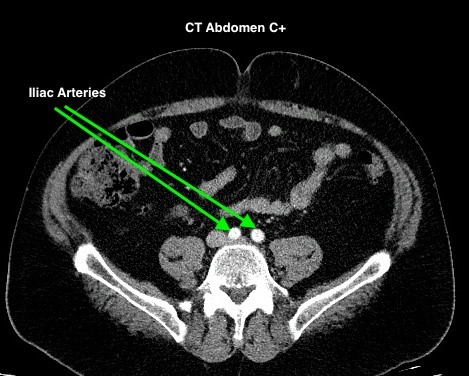
Timing Phase of Contrast Media for CT
The timing of image acquisition, relative to the time of injection of the contrast agent, can impact which anatomic structures harbour the greatest concentration of the administered contrast. This phenomenon is most important for CT. Five phases of contrast enhancement for CT imaging are discussed below.
The non-enhanced phase (no injected contrast) allows for a determination of the baseline status of the patient’s anatomy. Additionally, any structures that are calcified will be readily discernible, e.g. calculi, vascular calcifications, and dystrophic calcification in some tumours. These abnormalities will appear different from the surrounding tissues because of the calcium present in them. Administering contrast may obscure these calcified structures, especially urinary collecting system calculi, making detection difficult.
An early arterial phase, seconds after contrast administration, involves imaging very quickly after a bolus of intravenous contrast is administered, and can be useful for detecting certain abnormalities, specifically related to the arterial abnormalities (e.g. – arterial dissections).
The late arterial phase, requires images to be acquired 15 – 20 seconds after the early arterial phase has passed, and is ideal for imaging anatomic structures that are highly vascularized, such as the liver, spleen, and kidneys. These are especially useful for the identification of masses in these organs.
The portal venous phase, is a later phase image acquisition that takes advantage of the fact the contrast has cleared the arterial tree and is maximally concentrated in the mesenteric venous structures, the splenic vein, and the portal vein. This is often an important phase for assessing liver perfusion and cirrhotic patients for portal hypertension.
A delayed phase, also known as the ‘wash out phase’ or the ‘equilibrium phase’, occurs significantly later after contrast administration. This has also been called the total body opacification phase of contrast enhancement. The acquisition of serial images of the adrenal gland mass is a example of the use of ‘wash out’ imaging as benign lesions will demonstrate a different pattern of contrast ‘wash out’ than malignant tumours.
Timing of Contrast Media for CT:
- Non-Enhanced Phase – baseline status of patient’s anatomy, calculi, calcification
- Early Arterial Phase – for certain arterial abnormalities (e.g. arterial dissection)
- Late Arterial Phase – for highly vascularized structures (e.g. liver, spleen, kidney)
- Portal Venous Phase – concentrated in mesenteric venous structures
- Delayed Phase – wash out phase / equilibrium phase. To visualize lesions that retain contrast (e.g. tumors)
Digital Subtraction Angiography
Digital Subtraction Angiography (DSA) has a wide variety of indications, including, active GI bleeding, arterial stenosis for intervention, intervention for aneurysms, and arteriovenous malformation (AVM) diagnosis and treatment. Contrast for DSA is directly injected into the arterial lumen via a catheter that has been inserted by an Interventional radiologist. The images acquired for DSA are digitally subtracted using a computer to superimpose a non-enhanced image over the contrast enhanced images resulting in subtracted images that depict only the arteries as the background anatomy has been subtracted away.
Magnetic Resonance Imaging (MRI) Contrast
Contrast enhanced MR imaging is useful for a wide variety of applications, most notably to assess neurological malignancies. Other uses include characterizing lesions that have atypical appearances throughout the body, including neurologic abnormalities such as demyelination. It is also beneficial for assessing types of liver tumors and different types of arteriovenous malformations.
Risk Factors Associated with Contrast Agents
Any drug has risk associated with it, as does administering contrast agents for use in medical imaging. A potential acute situation related to water soluble contrast is the risk of an ‘allergic-like’ reaction (anaphylactoid). This reaction is anaphylactoid as it does not meet the defined criteria for a true allergic reaction (i.e. symptom free exposure to the antigen, development of an immune system mediated response to the antigen, anaphylactic reaction to the next exposure to the antigen). Anaphylactoid reactions to water soluble contrast agents can occur with the first exposure to contrast, skip contrast exposures, and can be highly unpredictable ranging from mild physical responses to cardiovascular collapse and death. Although severe anaphylactoid responses to water soluble contrast are very uncommon, a number of risk factors exist that increase a patient’s risk of developing an allergic-like reaction. The most significant risk factor for an anaphylactoid reaction, or any other type of reaction to water soluble contrast agent, is to have had a previous reaction to contrast. Furthermore, having a known allergy to anything else also increases a patient’s risk, but not nearly to the same extent as reacting to previous contrast. Therefore, contrast can likely still be administered if the patient reports an unrelated allergie.
For patients that are considered high-risk for an ‘allergic type’ adverse reaction to contrast, one may consider the use of premedication to lessen the chance of an adverse effect. The most common premedications used are oral corticosteroids supplemented with an oral, non-selective antihistamines. It is still possible that a patient can have an allergic-like or non-allergic type reaction, regardless of whether they have been administered premedication, and this is known as a “breakthrough reaction”. For more specific guidance about when to use premedication and for the recommended drug prophylactic regimen, please see the ACR Manual on Contrast Media.
There are other relative contraindications to contrast agents that should be taken into consideration including: Severe anxiety disorders; Patients on Beta-Blockers; Sickle-Cell Trait or Disease; and Hyperthyroidism (when using an iodinated contrast medium).
There are non-allergic adverse events that may effect the urinary system including, Contrast-Induced Nephrotoxicity (CIN), as well as Nephrogenic Systemic Fibrosis (NSF).
Contrast-Induced Nephrotoxicity is a direct result of the adverse effect that contrast agent have on glomerular filtration. There is often a transient reduction in renal function for any patient receiving iodinated, water soluble, contrast agents. This becomes more of an issue if the patient’s renal function is impaired prior to contrast injection. Often, it requires a clinical decision to proceed with contrast even when renal function is poor due to the importance of the imaging examination requested for patient management. The patient’s renal function can be protected slightly by aggressively hydrating the patient prior to contrast injection.
Nephrogenic Systemic Fibrosis (NSF) is related to the use of gadolinium based contrast agents for MRI and primarily involves the skin and the subcutaneous tissues, but can also affect organs (lungs, heart, skeletal muscles). The presentation initially involves skin thickening, and/or may involve pruritus. Note that NSF does not present acutely, and can take days-to-months to develop, and more rarely, years. The most significant risk factors for developing NSF include using GBCA and chronic/end-staged kidney disease. Other risk factors for NSF are patients that have experienced acute kidney injury (AKI), or have been exposed to high-doses of and/or had multiple exposures to GBCA.
There are newer, Group II, GCBAs that do not increase risk for NSF, however, Group I agents do increase risk and patients receiving group I agents should be screened because of the limited scientific research into the risks. Therefore, one way to mitigate the risks of NSF for a patient with end-stage renal disease that requires a contrast-enhanced MR investigation, it may be preferable to use an iodinated contrast media for a CT, or a type II GBCA instead.
Ultrasound Contrast Agents have the potential to cause direct toxic or allergic-like complications, however, this is a very infrequent risk. There may be an increased risk of cardiopulmonary reaction if the patient has a prior cardiopulmonary condition, however, these reactions are very rare.
Pregnancy and Breast Feeding
Caution needs to be taken when imaging women who are pregnant, or potentially pregnant, because of the potential risks associated with exposure of the fetus to ionizing radiation. If imaging is required despite concerns for radiation one must realize that water-soluble iodinated contrast agents and gadolinium contrast agents can cross the placenta, as well as, be excreted into breast milk. The effects of these drugs on the fetus, or infant, are not well understood.
While iodinated contrast agents administered intravenously are excreted into the breast milk, less than 1% of the administered dose will be excreted via this pathway, and subsequently less than 1% of the dose that the baby receives will be absorbed by the infant’s gastrointestinal tract. For Gadolinium contrast agents, less than 0.04% of the administered dose is excreted into the milk, and less than 1% of the ingested contrast is absorbed by the infant’s gastrointestinal tract. Therefore, the relative exposure of the infant to either of these contrast agents in minimal after a maternal administration. For more information on this topic refer to the ACR Manual on Contrast Media.
Risks of Contrast Agents:
- Potential for allergic-like reactions
- Higher risk with previous allergic-like reaction or unknown-type reaction to contrast agents
- Patients at high risk can take pre-medications to decrease their risk (corticosteroids, non-selective antihistamine)
- Consideration against using contrast agents: Severe anxiety; Patients on Beta-Blockers, Those with Sickle-Cell (trait or disease), and Hyperthyroidism
- Potential for Contrast-Induced Nephrotoxicity (CIN) or Nephrogenic Systemic Fibrosis (NSF)
Pregnancy and Breast Feeding
- Caution needs to be taken when imaging women who are pregnant or potentially pregnant
- Water-Soluble Iodinated agents and Gadolinium agents can cross placenta barriers and are excreted in breast milk
For More Information:
ACR Manual on Contrast – Website – https://www.acr.org/Quality-Safety/Resources/Contrast-Manual
Attributions
Fig 4.1A Fluoroscopy – Shoulder Arthrogram Early Phase of Injection by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
Fig 4.1B Shoulder Arthrogram Fluoroscopy Mid-Phase of Injection by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
Fig 4.1C Shoulder Arthrogram Late Phase of Injection by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
Fig 4.2A CT Aorta, C- by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
Fig 4.2B CT Iliac Arteries, C- by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
Fig 4.2C CT Aorta, C+ by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
Fig 4.2D CT Iliac Arteries, C+ by Dr. Brent Burbridge MD, FRCPC, University Medical Imaging Consultants, College of Medicine, University of Saskatchewan is used under a CC-BY-NC-SA 4.0 license.
