Chapter 17: Dietary Supplements
“Nutritional supplements are not a substitute for a nutritionally balanced diet.”
-Deepak Chopra, doctor, author, alternative medicine practitioner
Look around your home. Do you have any dietary supplements? It would not be surprising since about 77% of adults in the US report using dietary supplements.1 They are meant to “supplement the diet,” and it’s big business, generating more than $40 billion each year and providing over 50,000 products.2 They can come in pills, powders, tablets, liquids, sprays, and bars. Americans who use supplements regularly are more likely to report very good or excellent overall health, have a lower BMI, have health insurance, have higher socioeconomic levels, use alcohol moderately, refrain from smoking, and exercise more frequently than non-users. They are also more likely to be Asian, non-Hispanic, or white.1
Learning Objectives
- Define a dietary supplement and describe a typical user.
- Define ergogenics and provide possible mechanisms of action.
- Describe how dietary supplements are regulated in the US.
- Differentiate among the claims allowed on dietary supplements by FDA and the labeling requirements.
- Describe recommendations for choosing dietary supplements wisely.
17.1 Dietary Supplements: Definition, Regulation, Labeling
There are many reasons a person may choose to consume a dietary supplement. The most common reasons are to promote or maintain health, improve energy levels, boost the immune system, fill nutrient gaps, and/or support healthy aging.1 However, it is a perception of benefits, not quantifiable, research-based evidence, that appears to drive people to take supplements. In a recent survey, participants were asked “if a government-funded study denounced claims made by a supplement manufacturer,” 25% of respondents said they would disregard it. More than 80% said it was important to have access to supplements, and less than 25% are taking a supplement based on the advice of or under supervision of a health care professional.3 The most commonly consumed dietary supplements are micronutrients. Multivitamins (that contain multiple minerals as well) have been the most popular dietary supplement for decades, followed by vitamin D. Other popular supplements include probiotics, fiber, melatonin, and omega-3 fatty acids. A growing trend is the taking of herbals and botanicals like green tea, cannabidiol (CBD), and turmeric. Protein and amino acids remain the most popular dietary supplement for athletes.1
Many substances, objects, or treatments are ergogenic, meaning work enhancing. Ergogenic aids are substances, devices, practices, or treatments that improve athletic or physical performance. Forms of ergogenic aids include physiological, psychological, mechanical, pharmacological, and nutritional aids. Aids range from equipment such as a new type of golf club, racquet, or bat, or new performance fabric for exercise clothing, to substances ingested, injected, or snorted. Some ergogenic aids can have harmful side effects and some have been banned in professional sports. Ergogenic aids can come in many forms, ranging from very simplistic techniques or processes to drug supplementation. Dietary supplements are one type of ergogenic aid used extensively by both recreational and professional athletes in hopes of improving athletic performance. In general their purported benefits include improving athletic performance by acting as a stimulant on the central or peripheral nervous system, increasing the storage or availability of a nutrient used for energy production, reducing or neutralizing performance-inhibiting metabolic byproducts, or facilitating recovery.4
Regulation of Dietary Supplements
In the mid-1980s, as the science of nutrition was really getting started, businesses began to manufacture dietary supplements. By the mid-1990s it was a fairly large industry, and Congress was struggling to define whether or not these products were drugs, or whether they were something else. If they were to be considered drugs, they would be regulated by the Food and Drug Administration (FDA) as drugs. To get a new drug on the market, extensive testing on both animals and people under tightly controlled conditions is required to prove that they are safe, they work effectively, and that their health benefits outweigh their risks. Consumers generally feel confident that drugs that are approved by the FDA meet those requirements—some of the strictest in the world. It can take many years for a drug to be approved by the FDA which also regulates how it can be sold—either by prescription only or over-the-counter.5
The supplement manufacturing industry did not want their products to be considered drugs and lobbied Congress to be considered “food” instead. They argued that they were only selling substances that were already found in food, and food is not regulated, so they should not be either. In 1994, Congress agreed with the industry, deciding that these products were going to be considered food, defined them as “dietary supplements” and passed the Dietary Supplement Health and Education Act (DSHEA). DSHEA established the following:
- Dietary supplements are defined by the FDA as “a vitamin, mineral, herb, botanical, amino acid, metabolite, constitute, extract, or a combination of any of these ingredients” intended to be taken by mouth
- The manufacturer is responsible for ensuring that the product is safe, unadulterated, produced with good manufacturing practices (GMP), and properly and truthfully marked with a label
- Dietary ingredients in the supplement have to be federally regulated and Generally Recognized as Safe (GRAS)
- There is not a set limit on the amount of vitamins or minerals that can be put into a supplement
Contrary to popular perception, the FDA is not involved in pre-screening any dietary supplement for effectiveness or safety before it is sold to consumers. The FDA does not monitor for quality, purity, potency, or efficacy (does it work), nor does it regulate terms such as “natural,” “pure,” “quality assured,” or “clinically proven.” Supplement manufacturers are required to report any serious side effects associated with the product to FDA. Consumers can also report negative effects of supplements directly to the FDA, and if the FDA can prove that it is dangerous it can be restricted or removed from the market. This, however, has rarely occurred.
DSHEA also established the Office of Dietary Supplements (ODS) which is part of the National Institutes of Health (NIH). This government agency is tasked with conducting, supporting, and evaluating scientific research on the effectiveness of dietary supplements and communicating outcomes to the public. Their website is a great place to find credible information about a particular ingredient found in supplements, which may help you determine if it is right for you.
Advertising of dietary supplements is regulated by the Federal Trade Commission (FTC). Advertisements may not promote “pharmacological uses” of the product, and cannot use the words “cure” or “relieve” associated with any specific disease or condition. For example, a dietary supplement could not say it could “cure Alzheimer’s disease,” however, it could say that it “improves brain health.” These statements have not been evaluated by the FDA and often lack legitimate research to back them up.
Supplement: What’s in a Label?
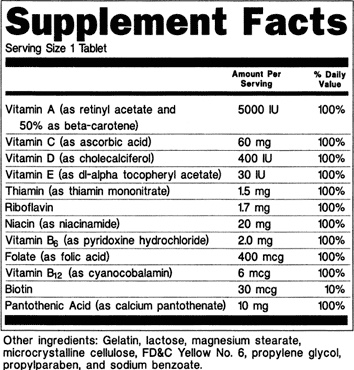
DSHEA established that dietary supplements must be “properly and truthfully labeled.” Labels must contain a statement of identity (the name of the dietary supplement), the net quantity of contents, a nutrition facts label similar to the one used on foods, an ingredient list, and the name and place of business of the manufacturer, packer, or distributor. Other required components of the label are country of origin, any allergens, and a disclaimer stating “these statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
Although the supplement facts label looks similar to those found on packaged foods, there are a few differences. Similarities include the listing of a serving size, servings per container, and % Daily Value (%DV) for any ingredients that have a RDA or AI like vitamins or minerals. However, dietary supplements can also list dietary ingredients that do not have a set recommendation, therefore no daily value. These ingredients could be botanicals like roots, flowers, or extracts.

An ingredient list is required, listed in descending order by weight. Dietary supplements must also list “inactive ingredients” such as those used in the tablets or capsules as fillers. You may also see cautionary statements about potential side effects or adulteration. However, if a label does not contain these statements it does not mean that the product is completely safe. Unlike conventional drugs, known possible adverse effects do not have to be listed on dietary supplement labels.
Proprietary Blends

You may see the term “proprietary blend” on a supplement facts label. It can also be called a “complex,” a “proprietary formula,” a “matrix”, or a “blend.” This is a list of ingredients that are part of a product formula specific to a particular manufacturer. The manufacturer must list total amount of substances in the proprietary blend and the individual ingredients, but not the amounts of each ingredient. In many cases consumers perceive the term to mean a higher quality product, although it has no bearing on supplement quality. Use of the term permits the manufacturer to withhold some ingredient information from users of the supplement. Be careful with proprietary blends, especially if you are a collegiate or professional athlete, or if you have any underlying health conditions. They could contain banned substances that are not listed on the label, or substances in amounts that could be harmful.
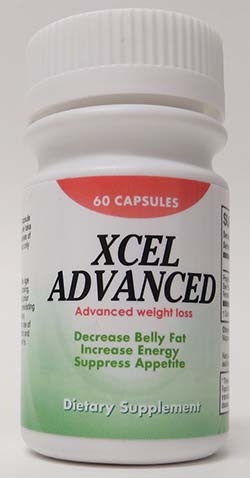
You may recall from Chapter 2 that the FDA allows three types of claims on packaged food products: health claims, nutrient claims, and structure/function claims. These may also be found on dietary supplements as long as they follow the same rules. Health and nutrient claims must meet FDA definitions in order to be used and require prior approval. Structure/function claims such as the “decrease belly fat,” “increase energy,” and “suppress appetite” on the supplement label in Figure 17.1.4, however, do not require prior approval and can be used as long as the product does not claim to treat, prevent, or cure any specific disease or condition. As with food packaging, structure/function claims can mislead a consumer into believing that the supplement has proven health benefits which is unlikely. These types of claims serve more as a marketing technique to try to sell consumers supplements they don’t need rather than actually providing health-related information for consumers. Structure/function claims are abundant on supplement labels, and the FDA disclaimer statement is required, although it is often less conspicuous on the label.
Supplement Quality
Because dietary supplements do not require FDA approval prior to sales, consumers rely on the manufacturer or distributor to make sure that the product is safe, efficacious (does what it claims), is not adulterated (contaminated with other ingredients), and contains the ingredients in the amounts on the supplement facts label. So how do we know if supplements on the market are pure and of high quality? Although there is no guarantee, there are third party entities that will verify the ingredients in a supplement for purity and potency. One such entity is the US Pharmacopeia (USP) who has the USP Dietary Supplements Verification Program, a voluntary process that supplement companies can utilize involving stringent testing to assess the quality of their manufacturing facilities, processes, and products.6

Other verification groups such as NSF International or Informed Sport, have developed independent testing standards and product certification for dietary supplements. Both groups focus on supplements for sport, verifying that they do not contain unsafe levels of contaminants, prohibited substances or masking agents, and that labels match what is actually in the product. If a dietary supplement has been verified by either of these organizations it will bear the respective certification mark (Figure 17.1.5).7,8 Consumer Lab also does independent third party testing of dietary supplements. They test for label accuracy, and compare multiple brands of the same type of supplement for quality. It is not supported by supplement manufacturers, but by consumers who subscribe for less than $50 per year.9,10
Adulteration of Supplements
Some dietary supplements have been adulterated meaning that the quality or effectiveness may be reduced because of the ingredients used. There are two types of adulteration seen in dietary supplements. The first is economic, a less expensive similar ingredient is used in place of the more expensive one listed on the label. The second is pharmaceutical, where an active drug is included in a “natural” dietary supplement.11 The FDA maintains a searchable public database where known adulterated supplements are listed. An analysis of this database found that, between 2007 and 2016, 746 supplements were adulterated with prescription drugs—some that had been recalled due to adverse effects and were no longer used, and some that had never been FDA approved. The types of drugs varied, but most often included weight loss pharmaceuticals, laxatives, designer steroids, and/or sexual enhancement drugs. About 20% contained two or more of these types of drugs. The FDA issued voluntary recalls for only 48% of them, the others remain on the market.12 It’s risky to ingest unknown pharmaceuticals, and there are 23,000 emergency room visits each year related to dietary supplement use.13 Do your homework prior to taking a dietary supplement. Do not assume “natural” means safe or even good. Some of the most toxic known substances are “natural.”
17.2 Choosing a Dietary Supplement
The good news about dietary supplements is that most are not harmful when taken at the recommended dosage. The bad news is that almost none of them have scientific evidence to support their efficacy. This means that Americans spend billions of dollars each year on something that has not been proven to be effective. Some may be adulterated by unsafe ingredients that can cause negative health effects as discussed previously, and others may have ingredients that interfere with other medications you may be taking. For example, taking the herbal supplement St. John’s Wort can speed the breakdown of many medications such as birth control pills, heart medications, and some antidepressants, reducing their effectiveness. Some antioxidant supplements may reduce the effectiveness of cancer chemotherapy.14
Adverse Effects
The law states that FDA does not have the authority to approve supplements or labeling before they are sold to consumers, and most are introduced to the market without notifying FDA. However, if a supplement causes harm, FDA can work with the manufacturer to modify it, or, in extreme cases, can remove it from the market. Therefore it is important to report any adverse effects (also known as side effects or bad reactions) that may occur after taking a dietary supplement to the FDA. Some may occur almost immediately after taking the supplement, while others may develop over time. Adverse effects can range from less serious reactions to life-threatening illness. Adverse events to watch for include itching, rash, hives, wheezing, fatigue, GI issues, difficulty urinating or changes in urine color, mood or behavioral changes, severe joint or muscle pain, blood in urine, vomit, or feces, mouth issues such as bleeding from the gums or tongue swelling, low blood pressure, fainting, chest pain, shortness of breath, and stroke. If you experience any of these symptoms, immediately stop using the product and seek medical care and advice. Then report the event to the FDA through their Safety Reporting Portal and/or through the FDA Safety Complaint Coordinator by calling the SAFEFOOD Information line, 1-800-SAFEFOOD (1-800-723-3366).15
Before choosing to take a dietary supplement, remember that although they may help you get adequate amounts of essential nutrients, if you don’t eat a nutritious variety of foods they cannot take the place of an overall healthy diet. Also keep in mind that manufacturers may fortify foods with vitamins, minerals, and other supplement ingredients, especially breakfast cereals and beverages. As a result, you may get more of these ingredients than you think, and more might not be better. Taking more than you need costs more and might also raise your risk of side effects, especially if you exceed the tolerable upper limit (UL) set by Dietary Reference Committees (see Appendix 3). For example, too much vitamin A can cause headaches, damage to your liver, reduce bone strength, and cause birth defects. Excess iron can cause nausea, vomiting, and may damage the liver and other organs. Some people need to be especially cautious about taking dietary supplements. Many supplements have not been well tested for safety in special populations like women who are pregnant or nursing, children, or those with underlying medical conditions.14

There are several things to consider prior to taking a dietary supplement. It is also recommended that you discuss any dietary supplements you’re thinking of taking with your healthcare provider.
- Check to see if the product has triggered health warnings or sanctions on the FDA web site mentioned above.
- Has the product been tested by independent labs (USP, NSF, Informed Sport)?
- Is the product “too good to be true?” Does it make unfounded, overzealous claims? Does it use medical jargon or scientific terms that a typical consumer may not understand? Does it use primarily testimonials such as before/after photos to tout the benefits?
- Is there scientific evidence to support the use of the supplement? You will need to do some research. There are reputable sources on the internet (Table 17.2.1) like the NIH ODS or the OPSS from the Department of Defense where you can find information about safety and efficacy (effectiveness), and dosage of supplements.
- Weigh the potential benefits with the potential risks. If you’re taking medications for a health condition, there could be negative side effects and interactions between the supplement and your medicines. If you’re looking for a health or energy boost, look at your diet first. Nature has “packaged” nutrients in foods better than we can, providing additional beneficial phytochemicals that we haven’t yet identified.
If you’re a collegiate or professional athlete, choosing a dietary supplement may be a little more complicated. Be sure to check with the governing body for your sport to determine if substances have been banned. The World Anti-Doping Agency (WADA) publishes a list every year of banned substances for athletes, and most organizations like the International Olympic Committee (IOC) and National Collegiate Athletic Association (NCAA) adopt this list for their athletes. Only use supplements that have been verified by a third party like NSF International or Informed Sport to be sure they are unadulterated and contain the ingredients in the correct dosage. And look at your diet first. Often dietary changes can provide the benefits you seek without the fear of disqualification due to the accidental consumption of a banned substance.
Table 17.2.1 Credible Sources of Dietary Supplement Information
| Organization | Site Name | Site URL |
| Center for Food Safety and Applied Nutrition (CFSAN), FDA | Dietary supplements | https://www.fda.gov/food/dietary-supplements |
| Consumer Lab | Independent supplement reviews
|
https://www.consumerlab.com/ |
| Department of Defense Operation Supplement Safety (OPSS) | Supplement resource for the military community
|
https://www.opss.org/ |
| Food and Nutrition Information Center (FNIC), National Agricultural Library (NAL), US Dept. of Agriculture (USDA) | Dietary supplements: Collection of resources on the topic | https://www.nal.usda.gov/fnic/dietary-supplements |
| Office of Dietary Supplements (ODS), NIH | Dietary supplements: Background information | https://ods.od.nih.gov/factsheets/DietarySupplements-HealthProfessional/ |
| Informed Sport | Independent reviews of dietary supplements | https://sport.wetestyoutrust.com/ |
| National Center for Complementary and Integrative Health (NCCIH), NIH | Using dietary supplements wisely | https://www.nccih.nih.gov/health/using-dietary-supplements-wisely |
| Memorial Sloan-Kettering Cancer Center (MSKCC) | About herbs, botanicals & other products | https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs |
| US Government Accountability Office (GAO) | Herbal dietary supplements: Examples of deceptive or questionable marketing practices and potentially dangerous advice | https://digital.library.unt.edu/ark:/67531/metadc290859/ |
| NSF International (NSF) | Independent reviews of dietary supplements | https://nsf.org |
| U.S. Pharmacopeia (USP) | Independent reviews of dietary supplements | https://www.quality-supplements.org/ |
| World Anti-doping Agency (WADA) | List of banned substances in athletic competition |
Key Takeaways
- The majority of Americans including members of the military consume a dietary supplement daily to improve or maintain health, fill nutrient gaps, or boost the immune system.
- Ergogenics are “work-enhancing” and range from dietary supplements to equipment and clothing.
- The Dietary Supplement Health Education Act of 1994 (DSHEA) defined dietary supplements and determined that the manufacturer, not FDA, is responsible for ensuring that the product is safe, unadulterated, produced with good manufacturing practices, and properly and truthfully marked with a label
- Dietary supplements must follow FDA guidelines for labeling including a required disclaimer that statements made by manufacturers have not been verified by FDA.
- FDA can fine and/or remove harmful dietary supplements from the market, but doesn’t do it often. Report all adverse effects to the FDA.
- Before choosing a dietary supplement, research it thoroughly, check for safety, and carefully consider cost versus benefits. Always try food first!
References:
- Council for Responsible Nutrition. (2019). Consumer survey. https://www.crnusa.org/2019survey/Topline-Infographic#more
- United States Food and Drug Administration. (2019, February 11). Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s new efforts to strengthen regulation of dietary supplements by modernizing and reforming FDA’s oversight. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-new-efforts-strengthen-regulation-dietary
- Bailey, R. L., Gahche, J. J., Miller, P. E., Thomas, P. R., & Dwyer, J. T. (2013). Why US adults use dietary supplements. JAMA Internal Medicine, 173(5), 355-361. https://doi.org/10.1001/jamainternmed.2013.2299
- Karpinski, C., & Rosenbloom C. A. (Eds.). (2017). Sports nutrition: A handbook for professionals (6th ed.). Academy of Nutrition and Dietetics.
- United States Food and Drug Administration. (2019, October, 28). Development and approval process/drugs. https://www.fda.gov/drugs/development-approval-process-drugs
- Eisenstein, M. (2019, January 10). Setting standards for supplements. Scientific American. https://www.scientificamerican.com/custom-media/setting-standards-for-supplements
- NSF International Certified for Sport. (n.d.). What our mark means. https://www.nsfsport.com/our-mark.php
- Informed Sport. (2021). Informed Sport Supplement Certification Process. https://sport.wetestyoutrust.com/about/certification-process
- Glassman, N. R. (2004). ConsumerLab.com. Journal of the Medical Library Association, 92(4), 509-510. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC521528/
- ConsumerLab.com. (n.d.). ConsumerLab.com. https://www.consumerlab.com/join/
- Cohen, P. (2018, October 12). The FDA and adulterated supplements – Dereliction of duty. JAMA Network Open, 1(6):e183329. doi:10.1001/jamanetworkopen.2018.3329
- Tucker, J., Fischer, T., Upjohn, L., Mazzera, D., & Kumar, M. (2018). Unapproved pharmaceutical ingredients included in dietary supplements associated with US Food and Drug Administration warnings. JAMA NetworkOpen, 1(6), e183337. doi:10.1001/jamanetworkopen.2018.3337
- Geller, A. I., Shehab, N., Weidle, N. J., Lovegrove, M. C., Wolpert, B. J., Timbo, B. B., Mozersky, R. P., & Budnitz, D. S. (2015). Emergency department visits for adverse events related to dietary supplements. New England Journal Medicine, 373(16), 1531-1540. doi:10.1056/NEJMsa1504267
- Office of Dietary Supplements. (2020, April 7). Dietary supplements: What you need to know. National Institutes of Health. https://ods.od.nih.gov/HealthInformation/DS_WhatYouNeedToKnow.aspx)
- United States Food and Drug Administration. (2022, May 25). Supplement Your Knowledge. https://fda.gov/food/information-consumers-using-dietary-supplements/supplement-your-knowledge
- Department of Defense. (2021). Operation Supplement Safety. https://www.opss.org/
Media Attributions
- 7E6A9C1C-6A20-44D1-A1F6-CCAE5FDAD21C_4_5005_c
- 30304A98-9A46-46C8-B6C0-5A0EB69DEFA1_4_5005_c
- AB279287-BCBE-4384-89F2-DCEC522BC809
- 324EE9F9-9C4E-4B8B-A0FA-800EB766827F_4_5005_c
- C9E9A918-FD07-451A-87EB-2002355AD026_4_5005_c
- 130DEC40-D0A7-40E2-A06F-5B7D5FBB2C61
substances, devices, practices, or treatments that improve athletic of physical performance
substituting a poorer quality, inferior ingredient or substance into a produce reducing the quality of the original product
