Chapter 13: Water
“If there is magic on this planet, it is contained in water.”
-Loren Eiseley (1907-1977), nature writer, educator, philosopher
Water is the foundation of all life—the surface of the earth is 70% water; the volume of water in humans is about 60%. Maintaining the right level of water in your body is crucial to survival, as either too little or too much will result in less-than-optimal functioning. One mechanism to help ensure the body maintains water balance is thirst. Thirst is the result of your body’s physiology telling your brain to initiate the thought to take a drink. Sensory proteins detect when your mouth is dry, your blood volume is too low, or blood electrolyte concentrations are too high and send signals to the brain stimulating the conscious feeling to drink. In this chapter, we will discuss the importance and functions of water in the human body and the consequences of getting too much or too little.
Learning Objectives
- Describe how body water is distributed.
- Describe the major aspects and organs involved in water regulation in the body.
- Explain the functions of water in the body.
- Discuss daily water gains and water losses including how the thirst mechanism works in the body.
- Discuss the consequences of too much or too little water.
- Describe how water is safe to drink and the pros and cons of bottled water.
13.1 Fluid Balance
Water is composed of two hydrogen (H2) atoms and one oxygen atom (O). A human body is primarily made up of water. An average adult body is about 60% water, containing 37-42 liters, weighing about 80 lb. Newborns are approximately 70% water. Fortunately, humans have compartmentalized tissues; otherwise we might just look like a water balloon! Adult males typically are composed of about 60% water and females are about 55% water. This gender difference reflects the differences in body fat content, since body fat is practically water free. This also means that if a person gains weight in the form of fat, the percentage of total body water content declines. As we age, total body water content also diminishes so that by the time we are in our 80s the amount of water in our bodies has decreased to around 45%. Does the loss in body water play a role in the aging process? Alas, no one knows. But, we do know that dehydration accelerates the aging process whereas keeping hydrated decreases headaches, muscle aches, and kidney stones.
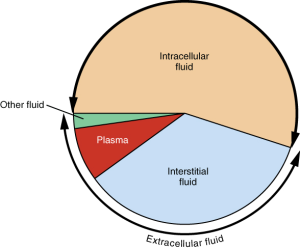
Although water makes up the largest percentage of body volume, it is not actually pure water but rather a mixture of cells, proteins, glucose, lipoproteins, electrolytes, and other substances. Electrolytes are substances that, when dissolved in water, dissociate into charged ions. Many minerals are also electrolytes. Positively charged electrolytes are called cations and negatively charged electrolytes are called anions. For example, in water, sodium chloride (the chemical name for table salt) dissociates into sodium cations (Na+) and chloride anions (Cl−). Solutes refers to all dissolved substances in a fluid, both charged, such as sodium (Na+), or uncharged, such as glucose. In the human body, water and solutes are distributed into two compartments: inside cells, called intracellular, and outside cells, called extracellular. The extracellular water compartment is subdivided into the spaces between cells also known as interstitial, blood plasma, and other bodily fluids such as the cerebrospinal fluid which surrounds and protects the brain and spinal cord.
Osmoregulation
One of the essential homeostatic functions of the body is to maintain fluid balance and the differences in solute composition between cells and their surrounding environment. Osmoregulation is the control of fluid balance and composition in the body. The processes involved keep fluids from becoming too diluted or too concentrated. Fluid compartments are separated by selectively permeable membranes, which allow some things, such as water, to move through while other substances cannot pass through or require special transport proteins, channels, and often energy (ATP). The movement of water between fluid compartments happens by osmosis, which is simply the movement of water through a selectively permeable membrane from an area where it is highly concentrated to an area where it is not so concentrated. Water is never transported actively; that is, it never takes energy for water to move between compartments. Although cells do not directly control water movement, they do control movement of electrolytes and other solutes and thus indirectly regulate water movement by controlling where there will be regions of high and low concentrations.
Cells maintain their water volume at a constant level, but the composition of solutes in a cell is in a continuous state of flux. This is because cells are bringing nutrients in, metabolizing them, and disposing of waste products. To maintain water balance a cell controls the movement of electrolytes to keep the total number of dissolved particles, called osmolality the same inside and outside (see Figure 13.1.3). The total number of dissolved substances is the same inside and outside a cell, but the composition of the fluids differs between compartments. For example, sodium exists in extracellular fluid at 14 times the concentration as compared to that inside a cell.
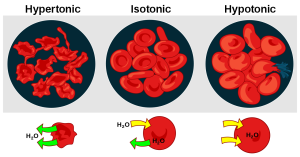
Cells maintain water volume by actively controlling electrolyte concentrations. Human erythrocytes (red blood cells) are shown in Figure 13.1.3. Three conditions are shown: hypertonic conditions (where the erythrocytes contract and appear spiky), isotonic conditions (where the erythrocytes appear normal), and hypotonic conditions (where the erythrocytes expand and become more round). If a cell is placed in a solution that contains fewer dissolved particles (hypotonic solution) than the cell itself, water moves into the more concentrated cell, causing it to swell. Alternatively, if a cell is placed in a solution that is more concentrated (known as a hypertonic solution) water moves from inside the cell to the outside, causing it to shrink. Cells keep their water volume constant by pumping electrolytes in and out in an effort to balance the concentrations of dissolved particles on either side of their membranes. When a solution contains an equal concentration of dissolved particles on either side of the membrane, it is known as an isotonic solution. Osmoregulation is critical to body functioning.
13.2 Functions of Water in the Body
You get up in the morning, use the toilet, take a shower, brush your teeth, drink, eat, drive, wash the grime from your windshield, get to work, and drink coffee. Next to a fountain you eat your lunch and drink a bottle of water, you use the toilet again, drive home, prepare dinner, etc. Add all the ways you use water every day and you still will not come close to the countless uses water has in the human body. Of all the nutrients, water is the most critical as its absence proves lethal within a few days. Organisms have adapted numerous mechanisms for water conservation. Water uses in the human body can be loosely categorized into four basic functions: vehicle for transportation, medium for chemical reactions, lubricant/shock absorber, and temperature regulator.
Water As a Transportation Vehicle
Water is called the “universal solvent” because more substances dissolve in it than any other fluid. Molecules dissolve in water because of the hydrogen and oxygen molecules’ ability to loosely bond with other molecules. Molecules of water (H2O) surround substances, suspending them in a sea of water molecules. The solvent action of water allows for substances to be more readily transported. A pile of undissolved salt would be difficult to move throughout tissues, as would a bubble of gas or a glob of fat. Blood, the primary transport fluid in the body, is about 78% water. Dissolved substances in blood include amino acids, lipoproteins, glucose, electrolytes, and metabolic waste products, such as carbon dioxide and urea. These substances are either dissolved in blood to be transported to cells to support basic functions or are transported away from cells to prevent waste build-up and toxicity. Blood is not just the primary vehicle of transport in the body, but also as a fluid tissue. Blood structurally supports blood vessels that would collapse in its absence. For example, the brain consists of 75% water which is used to provide structure.
Water As a Medium for Chemical Reactions
Water is required for even the most basic chemical reactions. Proteins fold into their functional shape based on how their amino acid sequences react with water. Enzymes must conduct their specific chemical reactions in a medium, which in all organisms is water. Water is an ideal medium for chemical reactions as it can store a large amount of heat, is electrically neutral, and has a pH of 7.0, meaning it is neutral (neither acidic nor basic). Additionally, water is involved in many enzymatic reactions as an agent to break bonds, or by its removal from a molecule, to form bonds. You may recall that when macromolecules are broken down during digestion via enzymes, water is added to the process to break the bond in a process called hydrolysis (hydro = water, lysis = break apart). When the body is building macromolecules such as proteins, amino acids are combined by removing water, a process called dehydration synthesis or condensation.
Water As a Lubricant/Shock Absorber
Many may view the slimy products of a sneeze as gross, but sneezing is essential for removing irritants and could not take place without water. Mucus is not only essential to discharge nasal irritants, but is also required for breathing, transportation of nutrients along the gastrointestinal tract, and elimination of waste materials through the rectum. Mucus is composed of more than 90% water and is a front-line defense against injury and foreign invaders. It protects tissues from irritants, entraps pathogens, and contains immune system cells that destroy pathogens. Water is also the main component of the lubricating fluid between joints and eases the movement of articulated bones.
The fluids that fill the extra space in the eyes and the cerebrospinal fluid surrounding the brain and spinal cord, are primarily water and buffer these organs against sudden changes in the environment. Watery fluids surrounding organs provide both chemical and mechanical protection. Just two weeks after fertilization, water fills the amniotic sac in a pregnant woman providing a cushion of protection for the developing embryo.
Water As a Temperature Regulator
Another homeostatic function of the body, termed thermoregulation, is to balance heat gain with heat loss and body water plays an important role in accomplishing this. Human life is supported within a narrow range of temperature, typically 96.2-99.1°F (35.7-37.3°C) with an average temp of 97.9°F (36.6°C).1 A body temperature too low or too high causes enzymes to stop functioning and metabolism is halted. At 82.4°F (28°C) muscle failure occurs and hypothermia sets in. At the opposite extreme of 111.2°F (44°C) the central nervous system fails and death results. Water is good at storing heat, an attribute referred to as heat capacity, and thus helps maintain the temperature set point of the body despite changes in the surrounding environment.
There are several mechanisms in place that move body water from place to place as a method to distribute heat in the body and equalize body temperature. The hypothalamus in the brain is the thermoregulatory center. The hypothalamus contains special protein sensors that detect blood temperature. The skin also contains temperature sensors that respond quickly to changes in immediate surroundings. In response to cold sensors in the skin, a neural signal is sent to the hypothalamus, which then sends a signal to smooth muscle tissue surrounding blood vessels causing them to constrict and reduce blood flow. This reduces heat lost to the environment. The hypothalamus also sends signals to muscles to erect hairs and shiver and to endocrine glands like the thyroid to secrete hormones capable of ramping up metabolism. These actions increase heat conservation and stimulate heat production in the body in response to cooling temperatures.
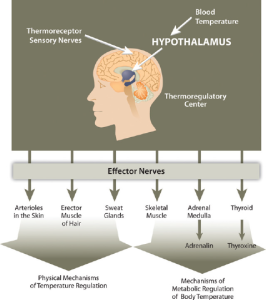
When the body generates heat and body temperature rises such as during exercise, being in a hot environment, or with a fever, the body dissipates this heat in a number of ways. Blood vessels under the skin dilate, increasing blood flow to the skin which is cooler than the internal body. The heat radiates from the skin to the environment. A second adaptation for body temperature regulation is evaporation. Sweat glands release sweat containing water and sodium. Remember that water has a high heat capacity, so when this sweat leaves the body via evaporation, it cools the body. A third mechanism for heat exchange is conduction which pulls heat from the body by transferring it to another object or body with which it is in contact such as a chair, towel, bandana, or another person. Of course conduction works both ways as the body can gain heat the same way. One additional way heat can be pulled from the body is with convection which occurs when air or water move across the skin. Think about how sitting under a fan or swimming in cold water can cool you on a hot day.
13.3 Regulation of Water in the Body
As you eat a bite of food, the salivary glands secrete saliva. As the food enters your stomach, gastric juice is secreted. As it enters the small intestine, pancreatic juice is secreted. Each of these fluids contains a great deal of water. How is that water replaced in these organs? What happens to the water now in the intestines? In a day, there is an exchange of about 10 liters of water among the body’s organs. The osmoregulation of this exchange involves complex communication between the brain, kidneys, and endocrine system. A homeostatic goal for a cell, a tissue, an organ, and an entire organism is to balance water output with water input.
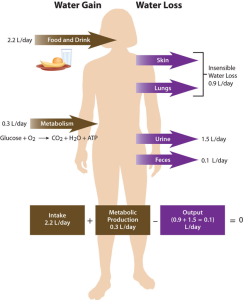
Regulation of Daily Water Input
Total water output per day averages 2.5 liters (about 10 cups). This must be balanced with water input. Our tissues produce around 300 ml (about 10 oz or 1.25 cups) of water per day through metabolic processes. The remainder of water output must be balanced by drinking fluids and eating solid foods. The average fluid consumption per day is 1.5 liters (about 6.25 cups), and water gained from solid foods is approximately 700 ml (24 oz or about 3 cups).
Dietary Gain of Water
The recommended AI for water is 3.7 liters (15.6 cups) for men and 2.7 liters (11.4 cups) for women. These intakes are higher than the average intake of 2.2 liters. It is important to note that the AI for water includes water from all dietary sources; that is, water coming from food as well as beverages. People are not expected to consume 11-16 cups of pure water per day. In America, approximately 20% of dietary water comes from solid foods (see Table 13.3.1). Beverages include water, tea, coffee, sodas, and juices. Please note that these recommended amounts are expected to meet the needs of healthy, sedentary people in temperate climates. Heat exposure, high levels of physical activity, illness, and other factors may increase one’s water needs.2
Table 13.3.1 Water Content in Foods
| Percentage | Foods |
| 90-99 | Nonfat milk, cantaloupe, strawberries, watermelon, lettuce, cabbage |
| 80-89 | Fruit juice, yogurt, apples, grapes, oranges, carrots, broccoli, pears |
| 70-79 | Bananas, avocados, cottage cheese, ricotta cheese, baked potato |
| 60-69 | Pasta, legumes, salmon, chicken breast |
| 50-59 | Ground beef, hot dogs, steak, feta cheese |
| 40-49 | Pizza |
| 30-39 | Cheddar cheese, bagels, bread |
| 20-29 | Pepperoni, cake, biscuits |
| 10-19 | Butter, margarine, raisins |
| 1-9 | Walnuts, crackers, cereals, pretzels, peanut butter |
| 0 | Oil |
There is some debate over the amount of water required to maintain health because there is no consistent scientific evidence proving that drinking a particular amount of water improves health or reduces the risk of disease. In fact, kidney stone prevention seems to be the only premise for water consumption recommendations. You may be surprised to find out that the commonly held belief that people need to drink eight 8 oz glasses of water per day isn’t an official recommendation and isn’t based on any scientific evidence! The amount of water/fluids a person should consume every day is actually quite variable and should be based on the climate a person lives in, as well as their age, physical activity level, and kidney function. No maximum for water intake has been set.
Thirst Mechanism: Why Do We Drink?
Thirst is an osmoregulatory mechanism to increase water input. The thirst mechanism is activated in response to changes in water volume in the blood, but is even more sensitive to changes in blood osmolality. Blood osmolality is primarily driven by the concentration of sodium cations. The urge to drink results from a complex interplay of hormones and neuronal responses that coordinate to increase water input and contribute toward fluid balance and composition in the body. As stated earlier, the “thirst center” is contained within the hypothalamus. In older people the thirst mechanism is less responsive and as we age there is a higher risk for dehydration. Thirst happens in the following sequence of physiological events:
- Receptor proteins in the kidney, heart, and hypothalamus detect decreased fluid volume and/or increased sodium concentration in the blood.
- Hormonal and neural messages are relayed to the brain’s thirst center in the hypothalamus.
- The hypothalamus sends neural signals to higher sensory areas in the cortex of the brain, stimulating the conscious thought to drink.
- Fluids are consumed.
- Receptors in the mouth and stomach detect mechanical movements involved with fluid ingestion.
- Neural signals are sent to the brain and the thirst mechanism is shut off.
The physiological control of thirst is the backup mechanism to increase water input. Fluid intake is controlled primarily by conscious eating and drinking habits dependent on social and cultural influences. For example, you might have a habit of drinking a glass of orange juice and eating a bowl of cereal every morning before school or work, or of drinking water throughout the day as you sit at your desk.
Regulation of Daily Water Output
As stated, daily water output averages 2.5 liters. The body loses about 400 ml (13 oz) of its daily water output through exhalation. Another 500 ml (17 oz) of water output occurs from the skin, and feces account for roughly 100 ml (3.4 oz) of water output. Urination provides the largest portion of daily water output, about 1500 ml (50 oz) per day. Regulating urine output is a primary function of the kidneys, and involves communication with the brain and endocrine system.
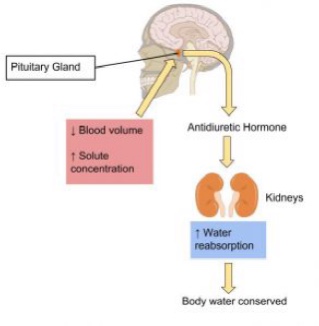
The kidneys are two bean-shaped organs, each about the size of a fist, located on either side of the spine just below the rib cage. The kidneys filter about 190 liters of blood and produce (on average) 1.5 liters of urine per day. Urine is mostly water, but it also contains electrolytes and waste products, such as urea. The amount of water filtered from the blood and excreted as urine is dependent on the amount of water in and the electrolyte composition of the blood.
Kidneys have protein sensors that detect blood volume from the pressure, or stretch, in the blood vessels of the kidneys. When blood volume is low, kidney cells detect decreased pressure and secrete the enzyme, renin. Renin travels in the blood and changes another protein into the active hormone, angiotensin. Angiotensin targets three different organs (the adrenal glands, the hypothalamus, and the muscle tissue surrounding the arteries) to rapidly restore blood volume and, consequently, blood pressure.
The Hypothalamus Detects Blood Osmolality
Sodium and fluid balance are intertwined. Osmoreceptors (specialized protein receptors) in the hypothalamus detect sodium concentration in the blood. In response to a high sodium level, the hypothalamus activates the thirst mechanism to encourage water consumption, and concurrently releases antidiuretic hormone (ADH) which reduces urine production and release. This increases fluid levels which increases blood volume, reducing sodium concentration. Kidneys can also stimulate ADH release in response to elevated sodium concentrations. This dual control of antidiuretic hormone release allows for the body to respond to both decreased blood volume and increased blood osmolality.
The Adrenal Glands Detect Blood Osmolality
Cells in the adrenal glands sense when sodium levels are low and potassium levels are high in the blood. In response to either stimulus, they release aldosterone. Aldosterone is released in response to angiotensin stimulation and is controlled by blood electrolyte concentrations. In either case, aldosterone communicates the same message, to increase sodium reabsorption and consequently water reabsorption. In exchange, for the reabsorption of sodium and water, potassium is excreted.
13.4 Consequences of Deficiency or Excess
As with all nutrients, having too much or too little water has health consequences.
Excessive water intake can dilute the levels of critical electrolytes in the blood. Water intoxication is rare, however when it does happen, it can be deadly. On the other hand, having too little water in the body is common. In fact, diarrhea induced dehydration is the number one cause of childhood death worldwide.
High-Hydration Status: Water Intoxication/Hyponatremia
Water intoxication mainly affects athletes who overhydrate. Water intoxication is extremely rare, primarily because healthy kidneys are capable of excreting up to one liter of excess water per hour. Overhydration was unfortunately demonstrated in 2007 by Jennifer Strange, who drank six liters of water in three hours while competing in a “Hold Your Wee for a Wii” radio contest. Afterward she complained of a headache, vomited, and died.3
Low-Hydration Status: Dehydration
Dehydration refers to water loss from the body without adequate replacement. It can result from either water loss or electrolyte imbalance, or most commonly, both. Dehydration can be caused by prolonged physical activity without adequate water intake, heat exposure, excessive weight loss, vomiting, diarrhea, blood loss, infectious diseases, malnutrition, electrolyte imbalances, and very high glucose levels. Physiologically, dehydration decreases blood volume. The water in cells moves into the blood to compensate for the low blood volume, and cells shrink. Signs and symptoms of dehydration include thirst, dizziness, fainting, headaches, low blood pressure, fatigue, low to no urine output, and in extreme cases, loss of consciousness and death. Signs and symptoms are usually noticeable after about 2% of total body water is lost.
Chronic dehydration is linked to higher incidences of some diseases. There is strong evidence that low hydration status increases the risk for kidney stones and exercise induced asthma. There is also some scientific evidence that chronic dehydration increases the risk for kidney disease, heart disease, and the development of hyperglycemia in people with diabetes. Older people often suffer from chronic dehydration as their thirst mechanism is no longer as sensitive as it used to be.
In the summer of 1965, the assistant football coach of the University of Florida Gators requested scientists affiliated with the university study why the withering heat of Florida caused so many heat-related illnesses in football players and provide a solution to increase athletic performance and recovery post-training or game. The discovery was that inadequate replenishment of fluids, carbohydrates, and electrolytes was the reason for the “wilting” of their football players. Based on their research, the scientists concocted a drink for the football players containing water, carbohydrates, and electrolytes and called it “Gatorade.” In the next football season the Gators were nine and two and won the Orange Bowl. The Gators’ success launched the sports drink industry, which is now a multibillion dollar industry that is still dominated by Gatorade.
Sports drinks are designed to rehydrate the body after excessive fluid depletion. Electrolytes in particular promote normal rehydration to prevent fatigue during physical exertion. Are they a good choice for achieving the recommended fluid intake? Are they performance and endurance enhancers like they claim? Who should drink them?
Typically, 8 oz of a sports drink provides between 50 and 80 kcals and 14 to 17 g of carbohydrate, mostly in the form of simple sugars. Sodium and potassium are the most commonly included electrolytes in sports drinks, with the levels of these in sports drinks being highly variable. The American College of Sports Medicine recommends a sports drink should contain 125 mg of sodium per 8 oz as it is helpful in replenishing some of the sodium lost in sweat and promotes fluid uptake in the small intestine, improving hydration. Check the labels of these products to determine if it meets your requirements. Chapter 12 contains information about making your own sports drink. Not every athlete requires a sport drink. Unless you are exercising for extended periods in hot, humid environments, most athletes can consume plain water for hydration and get their electrolytes in the food they eat.
Heat-Related Illnesses
As discussed in chapter 12, dehydration can lead to heat-related illness such as heat cramps, heat exhaustion, and heat stroke. The most severe is heat stroke, a life-threatening condition that occurs when the body temperature is greater than 105.1°F (40.6°C). It is the result of the body being unable to sufficiently cool itself by thermoregulatory mechanisms. Dehydration is a primary cause of heat stroke as there are not enough fluids in the body to maintain adequate sweat production, and cooling of the body is impaired. Signs and symptoms are dry skin (absence of sweating), dizziness, trouble breathing, rapid pulse, confusion, agitation, seizures, coma, and possibly death. Dehydration may be preceded by heat cramps or heat exhaustion, which is characterized by heavy sweating, rapid breathing, and fast pulse. The elderly, infants, and athletes are the most at risk for heat stroke.
Table 13.4.1 Heat-Related Illnesses
| Severity | Symptoms | Treatment | |
| Heat Cramps | Least severe | Muscle cramping | Fluids; stretching; low to moderate intensity movement |
| Heat Exhaustion | Moderately severe | Profuse sweating; cold, clammy skin; faintness; rapid pulse; low blood pressure | Cease activity; rest in shade/cooler place; fluids |
| Heat Stroke | Very Severe; life-threatening | Lack of sweat; dry, hot skin; lack of coordination; mental confusion; disorientation; loss of consciousness | Call 911; initiate body cooling (towels, ice packs, immerse or douse with cool water); give fluids only if victim is fully conscious, but no food |
13.5 Water Concerns
Sources of Drinking Water
In 1974, the US federal government enacted The Safe Drinking Water Act with the intention of providing the American public with safe drinking water. This act requires the Environmental Protection Agency (EPA) to set water quality standards including setting legal limits on 90 contaminants, and assure that the 150,000-plus public water systems in the country adhere to the standards. The list of regulated contaminants is revisited every 5 years.4 About 15% of Americans obtain drinking water from private wells, which are not subject to EPA standards. The well owner is responsible for the quality and safety of the water.5
Since the enactment of the Safe Drinking Water Act, drinking water supplies in the US have been some of the safest in the world. Producing water safe for drinking involves some or all of the following processes: screening out large objects, removing excess calcium carbonate from hard water sources, flocculation (which adds a precipitating agent to remove solid particles), clarification, sedimentation, filtration, and disinfection. These processes aim to remove unhealthy substances and produce high quality, colorless, odorless, good tasting water.6
Most drinking water is disinfected by the process of chlorination, which involves adding chlorine compounds to the water. Chlorination is cheap and effective at killing bacteria. However, it is less effective at removing protozoa, such as Giardia lamblia. Chlorine resistant protozoa and viruses are instead removed by extensive filtration methods. In the decades immediately following the implementation of water chlorination and disinfection methods in this country, waterborne illnesses, such as cholera and typhoid fever, essentially disappeared in the US. In fact, the treatment of drinking water is touted as one of the top public health achievements of the last century.7
Chlorine reaction with inadequately filtered water can result in the formation of potentially harmful substances. Some of these chlorinated compounds, when present at extremely high levels, have been shown to cause cancer in studies conducted in rodents. In addition to many other contaminants, the EPA has set maximum contaminant levels (legal threshold limits) for these chlorinated compounds in water, in order to guard against disease risk. The oversight of public water systems in this country is not perfect and waterborne illnesses are significantly under reported; however, there are far fewer cases of waterborne illnesses than those attributed to foodborne illnesses that have occurred in the recent past.
Hard vs Soft Water
Although water looks clear, it actually contains many solutes including mineral ions like calcium and magnesium. All water is naturally “hard” or “soft” based on its source. When water has high mineral content it is considered “hard.” The minerals accumulate in water that travels through limestone and chalk. Hard water often has a characteristic taste, and soap is less effective. In fact, the soap doesn’t foam up but instead forms a film which can be seen on glassware. The minerals can also accumulate in plumbing fixtures and appliances, reducing their effectiveness. “Soft” water has lower mineral content. Some water is naturally soft, but hard water can be “softened” by running it over an ion exchanger that changes calcium and magnesium for sodium. Softened water can sometimes taste a little salty, but soap is more effective, foaming without leaving a film. It is better for plumbing and appliances, and is better for skin and hair.
Is Bottled Water a Better Beverage Choice than Tap Water?
Bottled water is not regulated by the EPA but by the FDA. For bottled water, the FDA adheres to the same quality standards as those set by the EPA for tap water. Therefore, the contaminant levels set by the EPA for tap water are not different from bottled water, although there is much less testing, monitoring, and oversight of bottled water compared to tap water. Similar to tap water, the source and treatment of bottled water determines its taste and quality. Depending on where you live, tap water can be a dietary source of minerals (such as calcium and magnesium), but remember that food rather than water serves as a more prominent dietary source of these minerals.
Distilled and purified bottled waters have had all minerals removed during processing, but not all bottled waters have. Mineral waters, sparkling waters, and Artesian water are examples of bottled waters that contain some minerals, and are often more expensive. Tap water in the US costs, on average, much less than 1 cent per gallon, while bottled water is upwards of $4 per gallon. Moreover, bottled water uses more resources and produces much more waste in comparison to tap water. Iowa State University reports that in the US alone, the making of plastic bottles for bottled water consumption uses more than 1.5 million barrels of oil every year, which is more than the fuel required for 100,000 cars.8 There are times when consuming bottled water is essential. In times of natural or man made disasters, water supplies may become contaminated and bottled water may be the only safe alternative.
Table 13.5.1 Water Label Definitions9
| Label | Meaning |
| Artesian | Water from a confined aquifer |
| Mineral | Water containing >250 parts per million total dissolved solids, from protected underground water source (no minerals are added) |
| Purified | Water treated by distillation, deionization, reverse osmosis, or other processes. This bottled water is often from municipal water sources (i.e., tap water). |
| Spring | Water derived from an underground formation from which water flows naturally to the surface of the earth |
| Well | Water from a hole-bored, drilled, or otherwise constructed in the ground which taps the water from an aquifer |
Bottled Water vs Tap Water
Although many consumers believe that bottled water is more convenient and has a better taste than tap, there is no evidence that bottled water is safer or healthier. However, consuming bottled water is often a personal choice. In 2016, bottled water became the number one beverage (by volume) consumed in the US. In 2017, we drank 13.7 billion gallons of bottled water with sales topping $18.5 billion.10
The biggest issue with bottled water is the plastic bottles. Globally 1 million plastic beverage bottles per minute are sold. Less than 30% of plastic beverage bottles are recycled in the US, the rest end up in landfills.11 During the degradation process plastics are divided into smaller and smaller particles called microplastics which are less than 5 millimeters in size, about the length of a grain of rice. These microplastics are not only from single use plastic bottles, they are created during the breakdown of all plastics. They have been found in foods, drinking water (especially bottled water), clothing (nylon, spandex, polyester), and personal care products so they get into our systems in multiple ways. Some chemicals in plastics are hormone disruptors which have been linked to fertility issues, obesity, type 2 diabetes, and some forms of cancer.12
During the last few years the public has become more aware of the issue of plastic waste, and many ideas are brewing about how to either reduce their use or find new ways to deal with them once used. Some organizations, schools, and communities are banning single use plastic bottles. Other entrepreneurs are taking used bottles and turning them into clothing, shoes, printer ink cartridges, fence posts, decking material, and other consumer goods. However, it is easier and less expensive to make new plastic than it is to recycle,12 and scaling these processes to large populations is problematic. Other ideas are to have consumers pay the actual cost of delivery and recovery of the product11 or to make corporations more accountable for their packaging after consumers dispose of it.12 Either of these would significantly increase the cost of a bottle of water. It is unlikely that a single intervention will have an impact on the plastic waste associated with bottled water and other consumer goods. It will take many individual, local, and global efforts as worldwide we currently produce over 300 million metric tons of plastic each year.12
Key Takeaways
- A human adult body consists of 37 to 42 liters of water (80 lb). This water is a mix of cells, protein, glucose, electrolytes, and other solutes.
- Osmoregulation is the control of fluid balance and composition in the body.
- In the body, water functions as a transportation vehicle, a medium for chemical reactions, a lubricant/shock absorber, and a temperature regulator. The hypothalamus contains the thermoregulatory center.
- Daily water input should equal water losses. Daily water input is provided by food and drink, daily water output is regulated by kidneys, the hypothalamus, and the adrenal glands.
- Water intoxication can be life threatening as can dehydration which can lead to heat related illnesses such as heat stroke.
- The EPA regulates public drinking water sources in the US.
- Bottled water is regulated by the FDA. It is not safer or healthier than tap water, but is still a popular beverage choice. However, the plastic bottles water comes in may pose environmental and health issues.
Portions of this chapter were taken from OER Sources listed below:
Tharalson, J. (2019). Nutri300:Nutrition. https://med.libretexts.org/Courses/Sacremento_City_College/SSC%3A_Nutri_300_(Tharalson)
Titchenal, A., Calabrese, A., Gibby, C., Revilla, M.K.F., & Meinke, W. (2018). Human Nutrition. University of Hawai’i at Manoa Food Science and Human Nutrition Program Open Textbook. https://pressbooks.oer.hawaii.edu
Additional References:
- Obermeyer, Z., Samra, J. K., & Mullainathan, S. (2017). Individual differences in normal body temperature: longitudinal big data analysis of patient records. British Medical Journal, 359, j5468. https://doi.org/10.1136/bmj.j5468
- Institute of Medicine Food and Nutrition Board. (2004). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate.The National Academies Press.
- Katz, L. (2007). DJs axed after woman dies in Wii water-drinking contest. CNET. https://www.cnet.com/news/djs-axed-after-woman-dies-in-wii-water-drinking-contest-9678573/
- Environmental Protection Agency. (n.d.). Drinking water regulations. https://www.epa.gov/dwreginfo/drinking-water-regulations
- United States Geological Service. (n.d.). Domestic (private) supply wells. https://www.usgs.gov/mission-areas/water-resources/science/domestic-private-supply-wells?qt-science_center_objects=0#qt-science_center_objects
- Centers For Disease Control and Prevention. (2015, January 20). Water treatment. https://www.cdc.gov/healthywater/drinking/public/water_treatment.html
- Centers for Disease Control and Prevention. (2017, December 8). Disinfection with chlorine and chloramine. https://www.cdc.gov/healthywater/drinking/public/water_disinfection.html
- Litchfield, R. (2019). Bottled water: Know the facts. Iowa State University Extension and Outreach. http://www.extension.iastate.edu/publications/pm1813.pdf
- United States Food and Drug Administration. (2019, April 1). Bottled water everywhere: Keeping it safe. https://www.fda.gov/consumers/consumer-updates/bottled-water-everywhere-keeping-it-safe
- Arthur, R. (2018, June 1). ‘Bottled water is America’s favorite drink!’ Bottled water takes the top spot in the US. Beveragedaily.com. https://www.beveragedaily.com/Article/2018/06/01/Bottled-water-takes-top-spot-in-US-in-2017
- Parker, L. (2019, August 23). How the plastic bottle went from miracle container to hated garbage. National Geographic. https://www.nationalgeographic.com/environment/2019/08/plastic-bottles/
- Dow, C. (2021, June 4). Should you be concerned about microplastics? Nutrition Action Healthletter. https://www.nutritionaction.com/daily/food-safety/should-you-be-concerned-about-microplastics?/
Media Attributions
- B5A34C84-DB15-46BE-AE3E-5A8F905BB87F
- 38AC6B7A-6496-40AC-994C-86C2612A12FB
- Osmotic Pressure on Cells
- Thermoregulation
- 842B9ECC-0F45-47CE-8A80-BAFFF09D789E
- Hypophyse
- 8408901A-5CF1-4C1F-BBA6-38C019C6E6AC
positively charged electrolytes
negatively charged electrolytes
all dissolved substances in a fluid
within cells
outside of cells
space between cells
control of fluid balance in the body using solutes; keeps fluids from becoming too diluted or too concentrated
passive movement of fluids through a membrane from areas of higher concentration to areas of lower concentration
the concentration of dissolved particles (solutes) in a fluid
The process of chemically splitting macromolecules by the addition of water. It is used in many places in the body, including digestion where large carbohydrates, lipids, and proteins are split into smaller molecules.
Process of combining molecules by removing a water molecule. This occurs in many instances in the body including the building of macromolecules such as glycogen, storage fat, and protein.
balancing heat gain with heat loss to maintain homeostasis
