Chapter 16: Key Functions of Micronutrients (Bones, Blood, Antioxidants, Vision, Immunity)
“We are all sculptors and painters, and our material is our own flesh and blood and bone.”
-Henry David Thoreau (1817-1862), American naturalist, essayist, poet, and philosopher
This chapter will focus on the micronutrients necessary for structure and function of bones, teeth, and blood, those that serve as antioxidants, vitamin A and vision and the role micronutrients play in immunity.
Learning Objectives
- Describe the functions of calcium in the body, how calcium homeostasis is regulated, food sources of calcium, and effects of calcium deficiency and toxicity.
- Describe the synthesis, metabolism, and functions of vitamin D, as well as food sources and effects of deficiency and toxicity of vitamin D.
- Briefly describe the functions of phosphorus, magnesium, and fluoride in bone health and beyond, their food sources, and effects of deficiency and toxicity.
- Define osteoporosis, identify risk factors for development, and explain how osteoporosis can be prevented.
- Describe the role of blood, as well as the more specific functions, food sources, and effects of deficiency and toxicity for iron and vitamin K.
- Describe how free radicals develop and the role of antioxidants in fighting oxidative stress.
- Describe the general function of antioxidants, as well as the more specific functions, food sources, and effects of deficiency and toxicity for vitamin E, vitamin C, and selenium.
- Describe how vitamin A and beta-carotene contribute to normal vision, and know common food sources and effects of deficiency and toxicity of vitamin A.
16.1 Bones and Teeth
The human skeleton consists of 206 bones and other connective tissues that together support and protect many organs, produce red and white blood cells, and act as a storage depot for minerals such as calcium, phosphorus, and magnesium. Although bones may look inactive at first glance, they are living tissues that are dynamic and in a constant state of breaking down and rebuilding to withstand mechanical forces. Bones also contain a complex network of canals, blood vessels, and nerves that allow for nutrient transport and communication with other organ systems. In this section, we will look at how bone forms across the lifecycle and discuss the complex interactions of nutrients, hormones, genetics, and environmental factors that impact bone health.
Bones change in shape, size, and position throughout the life cycle. During infancy, childhood, and adolescence, bones are continuously growing and changing shape through two processes: growth (or ossification) and modeling. In the process of modeling, bone tissue is dismantled at one site and built up at a different site, which influences the shape of the bone. During childhood and adolescence, more bone is deposited than dismantled, with peak bone mass (maximum strength and density) being reached by age 30. Factors affecting peak bone mass include sex, race, hormones (e.g., estrogen and testosterone), nutrition (e.g., calcium and vitamin D intake), physical activity, and behavioral factors like smoking. These factors will be discussed in more detail later in the chapter.
The dynamic nature of bone means that new tissue is constantly formed, and old, injured, or unnecessary bone is dissolved for repair or for calcium release. The cell type responsible for bone resorption, or breakdown, is the osteoclast, while another type of cell, the osteoblast, is continually forming new bone.
There are several minerals and one vitamin involved in the structure and function of bone. Calcium and phosphorus together form the main mineral component of bone. Other minerals, including magnesium, fluoride, sodium, and potassium, play supporting roles. Vitamin D is the only vitamin that is directly involved in bone formation. This section focuses on these micronutrients (minus sodium and potassium which are covered in Chapter 15).
Calcium
Calcium is the most abundant mineral in the body and greater than 99% of it is stored in bone tissue. Although only 1% of the calcium in the human body is found in the blood and soft tissues, it is here that it performs the most critical functions. For example, calcium is required for the transmission of every nerve impulse, electrical signals sent from one nerve cell to another. It’s also required for every cycle of muscle contraction and relaxation including heartbeats. With inadequate calcium, muscles cannot relax, and instead become stiff and contract involuntarily, a condition known as tetany. Because your brain (nervous system) and heart (muscle) must work 24 hours per day, it is essential that blood calcium levels are maintained. Calcium is essential in blood clotting by activating clotting factors to fix damaged tissue. Calcium also plays vital roles in blood pressure regulation, enzyme activation, hormone secretion, and signaling between cells.1
The many roles of calcium around the body are critical to daily survival, so maintaining homeostasis, or steady state, of blood calcium levels is a high priority. Blood calcium levels are rigorously controlled in a very tight range. If blood calcium drops, your body initiates several mechanisms to restore homeostasis, including drawing calcium from the bone. While the calcium stored in bone is important for its long-term strength and structure, it also serves as a calcium reserve that can be drawn upon to support the vital functions of calcium in the body, should blood calcium drop too low. This is why bone health is dependent on the intake of dietary calcium and also why blood levels of calcium do not necessarily correspond to dietary intake.
Two endocrine glands are key players in the regulation of blood calcium concentrations: the thyroid gland and parathyroid glands. The thyroid gland, a small, butterfly-shaped gland located at the base of the neck, secretes a hormone called calcitonin. Four parathyroid glands, each about the size of a pea and located at the back of the thyroid gland, secrete a hormone called parathyroid hormone (PTH).
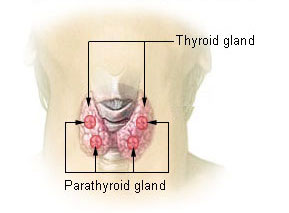
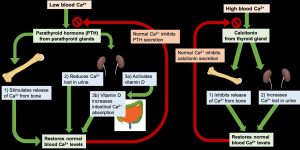
If the blood calcium concentration drops too low, the parathyroid glands release PTH, which acts in several ways to increase blood calcium levels:
- PTH stimulates the activity of osteoclasts to release calcium from bone.
- PTH acts on the kidney to reduce the amount of calcium lost in the urine, returning more to circulation.
- PTH stimulates enzymes in the kidney that convert vitamin D to its active form, also called calcitriol. Activated vitamin D acts on the intestine to increase the absorption of calcium. Vitamin D also works together with PTH to stimulate release of calcium from the bone and reduce calcium loss in urine.
Once blood calcium levels are normal, PTH levels drop, turning off all of these mechanisms of increasing calcium. On the other hand, if blood calcium levels become too high, the thyroid gland releases calcitonin, which lowers blood calcium levels by increasing calcium excretion in the urine, preventing further absorption of calcium in the gut and by directly inhibiting bone resorption. Once blood calcium concentration returns to normal, calcitonin levels decrease.
Through these two opposing pathways—PTH and vitamin D for raising blood calcium and calcitonin for lowering blood calcium—the body can very effectively maintain blood calcium homeostasis. This system is dependent upon stored calcium in bone, which is sacrificed when needed to ensure adequate blood calcium. In the short-term, this isn’t a problem, because bone remodeling allows you to replace calcium in the bone. However, in the long term, inadequate dietary calcium means you continuously draw down the calcium stores in your bones, resulting in declining bone mineral density and increased risk of fracture.
Calcium Sources and Requirements
In the typical American diet, calcium is obtained mostly from dairy products. A slice of cheddar or Swiss cheese contains over 200 mg of calcium. One cup of milk contains approximately 300 mg of calcium, which is about a third of the RDA for calcium for most adults. Foods fortified with calcium such as cereals, soy milk and other plant based beverages, and orange juice also provide approximately ⅓ of the calcium RDA. There are other good non-dairy sources of calcium, including some leafy green vegetables, legumes, soybeans (tofu, edamame, tempeh), and some seafood. Interestingly, the calcium in some vegetables such as kale, Brussels sprouts, and bok choy is better absorbed by the body than the calcium in dairy products where only about 30% of calcium is absorbed.
Calcium bioavailability can vary significantly. In general, calcium absorption is highest in infants and young children—who need relatively high amounts of calcium for building bone—and declines with age. As we get older, stomach acidity sometimes decreases, diarrhea occurs more often, kidney function is impaired, and vitamin D absorption and activation is compromised, all of which contribute to a decrease in calcium bioavailability. With higher calcium intake, especially from supplements, bioavailability decreases in order to prevent excessive calcium absorption. Some chemical components of plant foods bind to calcium and reduce bioavailability including oxalates in certain plants, the tannins in tea, phytates (found whole grains, beans, seeds, soy and nuts) and some fibers. Oxalates are found in high concentrations in spinach, sweet potatoes, cocoa, and beans. High-fiber, low-fat diets also decrease the amount of calcium absorbed, an effect likely related to how fiber and fat influence the amount of time food stays in the gut. Despite reduced absorption, these foods can still provide a significant amount of calcium.
The greatest positive influence on calcium absorption comes from having an adequate intake of vitamin D. People deficient in vitamin D absorb less than 15% of calcium from the foods they eat (more on vitamin D later). The hormone estrogen is another factor that enhances calcium bioavailability. Thus, as a woman ages and goes through menopause, during which estrogen levels fall, the amount of calcium absorbed decreases and the risk for bone disease increases. Some fibers, such as inulin, found in jicama, onions, and garlic, also promote calcium intestinal uptake.
Table 16.1.1 Calcium Content of Various Foods1
| Food | Serving | Calcium (mg) | %DV |
| Yogurt, low-fat | 1 c | 415 | 32 |
| Orange juice, calcium-fortified | 1 c | 349 | 29 |
| Mozzarella cheese, part skim | 1.5 oz | 333 | 26 |
| Sardines with bones, canned in oil | 3 oz | 325 | 25 |
| Cheddar cheese | 1.5 oz | 307 | 24 |
| Milk, nonfat | 1 c | 299 | 23 |
| Tofu, firm, made with calcium sulfate | ½ c | 253 | 19 |
| Cottage cheese (1%) | 1 c | 138 | 11 |
| Turnip greens, boiled | ½ c | 99 | 8 |
| Kale, cooked | 1 c | 94 | 7 |
The RDA for calcium for adults aged 19 to 50 is 1,000 mg/day. This number increases to 1,200 mg/day in women beginning at 51 years and men at 71 years. The UL for calcium, which applies to both food and supplements, is set at 2,500 mg/day for those between 19 to 50 years and decreases to 2,000 mg/day beginning at 51 years (for both men and women). Excessive calcium intake can lead to constipation, renal issues, vascular and soft tissue calcification, and kidney stones. However, consuming too much calcium from foods is rare and these are more likely to occur from use of supplements.1
In the short-term, there are no obvious signs of calcium deficiency. This is because the body stores so much calcium in bones, and just 1% of total body calcium is required for daily functioning. If acute low blood calcium does occur, symptoms include muscle cramping, numbness and tingling in fingers, convulsions, lethargy, poor appetite, and abnormal heart rhythms. Without treatment, this can lead to death.1
Much more common is a long-term calcium deficiency, resulting from a continuous draw of calcium stores from the bone. This causes osteopenia, or low bone mass, which can lead to osteoporosis if untreated. More about osteoporosis will be discussed later in the chapter.
Despite the wealth of evidence supporting the many health benefits of calcium (particularly bone health), the average American diet falls short of achieving the recommended dietary intakes of calcium. In fact, in females older than nine years of age, the average daily intake of calcium is only about 70% of the recommended intake. Here we will take a closer look at particular groups of people who may require extra calcium intake1:
- Adolescents. A calcium-deficient diet is common in teenagers (particularly girls) as their dairy consumption often drops considerably during adolescence.
- Amenorrheic women and the “female athlete triad.” Amenorrhea refers to the absence of a menstrual cycle. Women who fail to menstruate suffer from reduced estrogen levels, which can disrupt and have a negative impact on the calcium balance in their bodies. You may recall from Chapter 12 that the female athlete triad is a combination of three conditions characterized by amenorrhea, disordered eating patterns, and osteopenia.
- The elderly. As people age, calcium bioavailability is reduced, the kidneys lose their capacity to convert vitamin D to its most active form and are no longer efficient in retaining calcium, the skin is less effective at synthesizing vitamin D, there are changes in overall dietary patterns, and older people tend to get less exposure to sunlight.
- Postmenopausal women. Estrogen enhances calcium absorption. The decline in this hormone during and after menopause puts postmenopausal women especially at risk for calcium deficiency. Decreases in estrogen production are responsible for an increase in bone resorption and a decrease in calcium absorption.
- Lactose intolerant people. Groups of people, such as those who are lactose intolerant, or who adhere to diets that avoid dairy products, may not have an adequate calcium intake.
- Vegans. Vegans typically absorb reduced amounts of calcium because their diets favor plant-based foods that contain oxalates and phytates.
*If you are lactose intolerant, have a milk allergy, and/or are a vegan, remember that there are several lactose-free dairy products on the market and many plant-based foods that have a good amount of calcium.
Calcium Supplements: Which One to Buy?
Many people choose to fulfill their daily calcium requirements by taking calcium supplements. Calcium supplements are sold primarily as calcium carbonate, calcium citrate, calcium lactate, and calcium phosphate, with elemental calcium contents of about 200 mg per pill. It is important to note that calcium carbonate requires an acidic environment in the stomach to be used effectively. Although this is not a problem for most people, it may be for those on medication to reduce stomach acid production or for the elderly who may have a reduced ability to secrete acid in the stomach. For these people, calcium citrate may be a better choice. Otherwise, calcium carbonate is the cheapest. The body is capable of absorbing approximately 30% of the calcium from these forms.
Beware of Lead
There is public health concern about the lead content of some brands of calcium supplements. Supplements derived from natural sources such as oyster shell, bone meal, and dolomite (a type of rock containing calcium magnesium carbonate) should be avoided as they are known to contain high amounts of lead. Because lead levels in supplements are not disclosed on labels, it is important to know that products not derived from oyster shell or other natural substances are generally low in lead content.
Diet, Supplements, and Chelated Supplements
In general, calcium supplements are less likely to provide health benefits linked to higher calcium intake than dietary sources of calcium. This is partly attributed to the fact that dietary sources of calcium supply additional nutrients with health-promoting activities. It is reported that chelated forms of calcium supplements are easier to absorb as the chelation process protects the calcium from oxalates and phytates that may bind with the calcium in the intestines. However, these are more expensive supplements and may only increase calcium absorption up to 10%. In people with low dietary intakes of calcium, calcium supplements have a negligible benefit on bone health in the absence of a vitamin D supplement.
Vitamin D
Vitamin D refers to a group of fat-soluble vitamins derived from cholesterol. Vitamins D2 (ergocalciferol) and D3 (calcitriol) are the only ones known to have biological actions in the human body. Vitamin D was discovered in 1919 and is unique in that the skin can synthesize it when exposed to sunlight; hence it is often called the “sunshine vitamin.”2
Vitamin D synthesis in the skin begins with the conversion of cholesterol to 7-dehydrocholesterol. Then, in the presence of ultraviolet (UV) rays from sunlight, 7-dehydrocholesterol is converted to vitamin D3, which is transported to the liver by a binding protein. Vitamins D2 and D3 are both inactive until they undergo two hydroxylations—chemical reactions that add a hydroxyl (-OH) group. The first hydroxylation occurs in the liver, creating calcidiol. This is the circulating form of vitamin D and the form measured in blood to assess a person’s vitamin D status. The second hydroxylation occurs in the kidneys and forms calcitriol, the biologically active form of vitamin D.
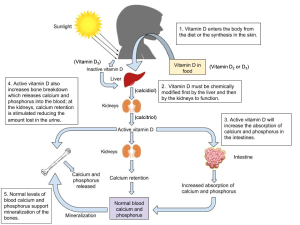
Recall from our discussion of blood calcium regulation earlier that one of the actions of PTH is to stimulate enzymes in the kidney that perform this last step in the activation of vitamin D. Active vitamin D increases the absorption of both calcium and phosphorus in the intestine, as well as working with PTH to reduce calcium loss in the urine and stimulate release of calcium and phosphorus from the bone. In these ways, vitamin D plays a critical role in both maintaining blood calcium homeostasis and enhancing the supply of calcium and phosphorus for bone mineralization. Vitamin D deficiency results in poor bone mineralization, with serious consequences in both children and adults, as we’ll discuss later in this chapter.
Beyond its role in bone health, vitamin D has many other functions in the body. Cells throughout the body have vitamin D receptors in their nuclei, and by binding to these receptors, vitamin D is thought to regulate the expression of hundreds of genes. Specifically, vitamin D is known to play important roles in regulating cellular differentiation and growth, immunity, insulin secretion, and blood pressure. Studies have found correlations between low circulating vitamin D levels and increased risks of chronic diseases including cancer, diabetes, cardiovascular disease, and multiple sclerosis. However, correlation does not equal cause and chronic diseases such as these are typically multifactorial in nature, so research in this area is ongoing.3
In most people, vitamin D synthesis in the skin provides a significant portion of their body’s needs, and a little sun exposure can go a long way. Vitamin D researchers suggest that most people need between 5 and 30 minutes of sun exposure between 10 AM and 4 PM, at least twice per week, in order to synthesize adequate vitamin D. However, any factors that decrease exposure to UV rays can interfere with vitamin D synthesis, including the following4:
- Geographic latitude and season. Exposure to UV light is greatest at the equator and declines as you move further north or south. Likewise, in the summer months, the sun is directly overhead for a greater part of the day, so you have more opportunities to synthesize vitamin D. In the winter, the sun stays lower in the sky, day length is shorter, and cloud cover is more likely to block the sun’s rays, all of which decrease opportunities to synthesize vitamin D. At higher latitudes, vitamin D synthesis is inadequate for at least a few months during the winter, because the sun simply doesn’t get high enough in the sky to provide enough UV radiation on earth’s surface. Ozone and air pollution can also block UV rays and decrease vitamin D synthesis.
- Skin pigmentation. Darker skin pigmentation, caused by greater melanin production in the skin, decreases UV light absorption. This helps to protect the skin from damage from UV radiation—a helpful adaptation for those living closer to the equator—but it also reduces synthesis of vitamin D. People with darker skin pigmentation need to spend more time in the sun in order to synthesize the same amount of vitamin D as those with lighter skin.
- Age. The efficiency of vitamin D synthesis declines with age. In addition, older adults often spend less time outside so may receive less exposure to sunlight.
- Sun-protective behavior. While some UV light exposure is needed to synthesize vitamin D, UV radiation is also carcinogenic, and too much exposure increases the risk of skin cancer. It’s wise to protect your skin from UV radiation by applying sunscreen, covering up with clothing and a hat, finding shade, and avoiding sun exposure in the middle of the day. People who are highly vigilant in these sun-protective behaviors or simply aren’t able to go outside during the day may not get enough UV light for vitamin D synthesis.
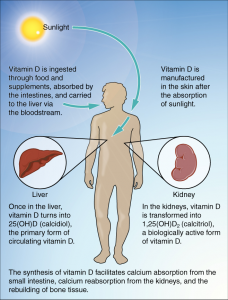
With so many factors affecting UV radiation exposure, many people are unable to synthesize enough vitamin D for at least part of the year. Because vitamin D is fat-soluble, liver and adipose storage can supply the body for a while. Beyond that, dietary sources and supplements may be needed to meet the vitamin D requirement.4
Vitamin D Sources and Requirements
Only a few foods are naturally good sources of vitamin D. These include fatty fish such as salmon, tuna, and mackerel, as well as fish liver oil (e.g., cod liver oil). Smaller amounts are found in egg yolks, cheese, and beef liver. Additionally, some mushrooms grown in UV light can be a good source of vitamin D. Most cow’s milk is fortified with vitamin D in the US but other dairy products such as ice cream and cheese are not. Fortified orange juice, soy milk and other plant-based beverages, and breakfast cereal can all contribute to dietary intake of vitamin D, although amounts added vary significantly between products.4
Table 16.1.2 Vitamin D Content of Various Foods4
| Food | Serving | Vitamin D (mcg) | %DV |
| Cod liver oil | 1 tbsp | 34.0 | 170 |
| Rainbow trout, farmed, cooked | 3 oz | 16.2 | 81 |
| Salmon, sockeye, cooked | 3 oz | 14.2 | 71 |
| White mushrooms, raw | ½ c | 9.2 | 46 |
| Milk (2%), vitamin D-fortified | 1 c | 2.9 | 15 |
| Atlanta sardines, canned in oil | 2 each | 1.2 | 6 |
| Egg, scrambled | 1 large | 1.1 | 6 |
| Tuna fish, canned in water | 3 oz | 1.0 | 5 |
| Cheddar cheese | 1 oz | 0.3 | 2 |
| Chicken breast, roasted | 3 oz | 0.1 | 1 |
The RDA for vitamin D is 15 mcg/day (or 600 IU) for men and women. The UL for vitamin D is 1,000 mcg/day (4000 IU) for supplemental intake. Vitamin D toxicity is rare but can also raise blood levels of calcium leading to increased risk of kidney stones and more seriously, vascular and tissue calcification.4

A deficiency of vitamin D in children can lead to rickets. This is characterized by soft, weak, deformed bones that are exceptionally susceptible to fracture. Rickets was common in the US until the 1930s, when milk processors were asked to add vitamin D to cow’s milk, virtually eliminating the condition. However, rickets does still occur in breastfed infants (who are not provided supplemental vitamin D) and children raised on vegan diets who aren’t provided with other sources of vitamin D, particularly if they have darker skin pigmentation.5
In adults, vitamin D deficiency causes a similar disease called osteomalacia, which is characterized by low bone density. Osteomalacia has the same symptoms and consequences as osteoporosis and often coexists with osteoporosis. Vitamin D deficiency is common, especially in the elderly population, darker skinned populations, and in the many people who live in the northern latitudes where sunlight exposure is much decreased during the long winter season.
Phosphorus
Phosphorus is the second most abundant mineral in the human body, and 85% of the body’s phosphorus is housed within the skeleton. In addition, phosphorus (in the form of phosphate) is a structural component of cell membranes (as phospholipids), RNA and DNA, and adenosine triphosphate (ATP). Phosphate also plays important roles in regulating cell signaling, enzyme activity, and acid-base balance, as well as being part of creatine phosphate, an energy source for muscles during exercise.6
Because phosphorus is present with calcium in mineralized bone, it is somewhat regulated in parallel to calcium. PTH and activated vitamin D stimulate bone resorption, increasing not only blood levels of calcium, but also blood phosphate levels. However, in contrast to the effect of PTH on calcium reabsorption by the kidney, PTH stimulates the renal excretion of phosphate so that it does not accumulate to toxic levels.
Table 16.1.3 Phosphorus Content of Various Foods7
| Food | Serving | Phosphorus (mg) | %DV |
| Yogurt, low-fat | 6 oz | 245 | 18 |
| Atlantic salmon, farmed, cooked | 3 oz | 214 | 17 |
| Chicken breast, roasted | 3 oz | 182 | 15 |
| Lentils, boiled | ½ c | 178 | 14 |
| Mozzarella cheese, part skim | 1.5 oz | 197 | 16 |
| Cashew nuts, roasted | 1 oz | 139 | 11 |
| Russet potato, baked | 1 medium | 123 | 10 |
| Peas, green, boiled | ½ c | 94 | 8 |
| Oatmeal, cooked with water | ½ c | 90 | 7 |
| Egg, hard boiled | 1 large | 86 | 7 |
The RDA for phosphorus for adults is 700 mg/day for men and women. The UL for phosphorus is set at 4,000 mg/day and applies to food and supplements. While toxicity is rare, excessive phosphorus supplementation can interfere with calcium regulation and cause calcification, or hardening of soft tissues, especially the kidneys.7
Most Americans are not at risk for having a phosphate deficiency. It is present in many foods popular in the American diet including meat, fish, dairy products, processed foods, and beverages. It is also added to many foods because it acts as an emulsifying agent, prevents clumping, improves texture and taste, and extends shelf life. The average intake of phosphorus in US adults ranges between 1,000 and 1,500 mg/day, well above the RDA of 700 mg/day, but well below the UL.
Magnesium
Approximately 60% of magnesium in the human body is stored in the skeleton, making up about 1% of mineralized bone tissue. Observational studies link magnesium deficiency with an increased risk for osteoporosis. A magnesium deficient diet is associated with decreased levels of PTH and activation of vitamin D, which may lead to an impairment of bone remodeling. Only a few clinical trials have evaluated the effects of magnesium supplements on bone health and their results suggest some modest benefits on bone mineral density.
In addition to participating in bone maintenance, magnesium has several other functions in the body. In every reaction involving the cellular energy molecule (ATP), magnesium is required. Additionally, more than 300 enzymatic reactions require magnesium. It plays a role in the synthesis of protein, DNA, and RNA, is essential for nerve conduction, muscle contraction, and blood glucose control. Another health benefit of magnesium is that it may decrease blood pressure.
Magnesium Sources and Requirements
Magnesium is part of the green pigment, chlorophyll, which is vital for photosynthesis in plants; therefore, green leafy vegetables are a good dietary source for magnesium. Magnesium is also found in high concentrations in fish, dairy products, meats, whole grains, and nuts. Additionally chocolate, coffee, and hard water contain a good amount of magnesium. Typically, Western diets lean toward low fish intake and an unbalanced consumption of refined grains over whole grains. Therefore, most people in America do not meet the RDA for magnesium in their diets.
Table 16.1.4 Magnesium Content of Various Foods8
| Food | Serving | Magnesium (mg) | %DV |
| Almonds, roasted | 1 oz | 80 | 19 |
| Spinach, boiled | ½ c | 78 | 19 |
| Cashew nuts, roasted | 1 oz | 74 | 18 |
| Soymilk | 1 c | 61 | 15 |
| Black beans, cooked | ½ c | 60 | 14 |
| Edamame, shelled, cooked | ½ c | 50 | 12 |
| Peanut butter, creamy | 2 tbsp | 49 | 12 |
| Potato, baked | 3.5 oz | 43 | 10 |
| Banana | 1 medium | 32 | 8 |
| Avocado | ½ c | 22 | 5 |
The RDA for magnesium for adults age 19 to 30 is 400 mg/day for men and 310 mg/day for women. After age 30, this increases to 420 mg/day for men and 320 mg/day for women. The UL for magnesium is set at 350 mg/day and applies only to supplements (as this amount is more than the RDA for women). Too much magnesium from food does not pose a health risk in healthy individuals as the kidneys eliminate excess amounts in the urine. Symptoms of excessive magnesium supplementation can include hypotension (low blood pressure), nausea, vomiting, facial flushing, and lethargy before progressing to muscle weakness, difficulty breathing, irregular heartbeat, and cardiac arrest.8
Obvious magnesium deficiency due to low dietary intake is rare in healthy people, because the kidneys can decrease urinary excretion of this mineral when intake is inadequate. However, those at greater risk of magnesium deficiency include people with type 2 diabetes, gastrointestinal diseases like Crohn’s and celiac, chronic alcoholism, and older adults. Symptoms may include decreased appetite, nausea, vomiting, fatigue, and weakness. If extreme, it can cause personality changes, muscle cramps, numbness, tingling, seizures, and an abnormal heart rhythm.8
Fluoride
Fluoride is known mostly as the trace mineral that combats tooth decay. It assists in tooth and bone development and maintenance. Because it isn’t necessary for growth or to sustain life, fluoride is generally not considered an essential mineral. However, fluoride’s role in preventing dental caries (i.e., tooth decay), the most prevalent chronic disease in children and adults, underscores the importance of this mineral in the human diet.9 Fluoride combats tooth decay via three mechanisms:
- Blocking acid formation by bacteria
- Preventing demineralization of teeth
- Enhancing remineralization of destroyed enamel
As a natural mineral, fluoride is present in the soil and water in varying concentrations depending on geographical location. In the 1930s, researchers observed that children living in areas with naturally higher fluoride concentrations in their drinking water had a lower incidence of cavities, leading to the idea that adding fluoride to municipal water supplies could benefit public health. Fluoride was first added to drinking water in 1945 in Grand Rapids, Michigan. Now over 60% of the US population consumes fluoridated drinking water. It is estimated that fluoridated water prevents approximately 25% of cavities in children and adults. It is recommended by the American Dental Association, American Academy of Pediatrics, US Public Health Service, and World Health Organization (WHO). The Centers for Disease Control and Prevention considers water fluoridation one of the ten great public health achievements in the twentieth century.10
The optimal fluoride concentration in water to prevent tooth decay ranges between 0.7-1.2 mg per liter. Exposure to fluoride at 3-5 times this concentration before age 8 and the growth of permanent teeth can cause fluorosis, which is the mottling and discoloring of the teeth. When brushing the teeth of children younger than 2 years, it is recommended to use a child-sized soft bristled toothbrush and fluoridated toothpaste the size of a grain of rice. Because fluoridated oral care products often taste good, making them appealing to young children, it is important to make sure they do not consume too much fluoride by swallowing toothpaste or other oral care products. In some circumstances, based on the level of fluoride in community drinking water and other sources of fluoride, you may choose a non-fluoridated toothpaste. Check with your dentist. When assessing the risks and benefits, determine if the child may be at high risk for tooth decay because of factors such as poor hygiene, poor diet, or history of decay in the child, and in their siblings or parents. Fluoride supplements should be considered carefully.11
Fluoride’s benefits to teeth are well substantiated, but fluoride also plays an important role in the mineralization of bones, increasing their structural stability. Fluoride is currently being researched as a potential treatment for osteoporosis. The data are inconsistent on whether consuming fluoridated water reduces the incidence of osteoporosis and fracture risk. Fluoride does stimulate osteoblast bone building activity, and fluoride therapy in patients with osteoporosis has been shown to increase bone mineral density. In general, it appears that at low doses, fluoride treatment increases bone mineral density in people with osteoporosis and is more effective in increasing bone quality when the intakes of calcium and vitamin D are adequate. However, the doses of fluoride used to treat osteoporosis are much greater than that in fluoridated water.
Greater than 70% of a person’s fluoride comes from drinking fluoridated water when they live in a community that fluoridates the drinking water. Other beverages with a high amount of fluoride include teas and grape juice. Solid foods do not contain a large amount of fluoride. Fluoride content in foods depends on whether it was grown in soils and water that contained fluoride or cooked with fluoridated water. Canned meats and fish that contain bones do contain some fluoride.
The AI for fluoride is 4 mg per day for men and 3 mg per day for women. The UL for fluoride in adults is 10 mg, however the period of greatest concern for fluorosis occurs prior to the age of 8.12
Additional Micronutrients that contribute to Bone Health
While calcium, vitamin D, phosphorus, magnesium, and fluoride are closely linked with bone health, other micronutrients are also involved. Vitamin C and copper are necessary for enzymes that synthesize collagen which provides strength to bone. Vitamin K and manganese are also required for the synthesis of non-collagen bone proteins.13
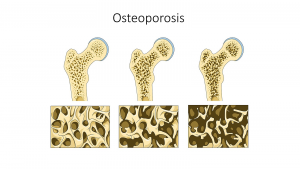
Osteoporosis
Osteoporosis is a bone disease that occurs when bone density or bone mass decreases. The bone becomes thinner and more porous and is therefore more susceptible to breaking. A precursor to osteoporosis, called osteopenia, describes bone density that is lower than recommended, but not yet at the osteoporotic level. Osteporosis/osteopenia are considered a silent disease because one does not feel their bones weakening. It can go undetected until bones become so weak that they fracture due to a sudden bump or fall. Other signs may include loss of body height and curvature of the upper back. According to the National Institute of Arthritis and Musculoskeletal and Skin Diseases, more than 53 million people in the US either have osteoporosis already or are at high risk of developing it due to low bone mass (osteopenia).14 Nearly 1 in 2 women and up to 1 in 4 men, aged 50 or older are at risk of an osteoporotic bone fracture. The most common sites of fracture include hip, spine, or wrist. More than 20% of older adults who break a hip die within one year, either from complications of the break itself or the surgery to repair it. Most will require long-term nursing home care.
How is bone health assessed? A bone mineral density (BMD) test can detect osteopenia/osteoporosis and predict the risk of bone fracture. The most common tool used to measure BMD is called dual energy X-ray absorptiometry (DEXA) (previously described in Chapter 9). This method can measure bone density over the entire body, but most often the DEXA scan focuses on measuring BMD in the hip and the spine. It is recommended that women should have their BMD assessed beginning at 65 years old or younger if they are at increased risk of osteoporosis.15
An individual’s chance of developing osteoporosis depends on several risk factors, some of which are controllable and some of which are not. It is thought that genetic factors (such as sex and race) may account for up to 75% of bone mass, while lifestyle factors (such as diet and exercise habits) account for the remaining 25%.16
Osteoporosis risk factors that cannot be controlled include:
- Body frame size. People with small frames are at higher risk for osteoporosis.
- Ethnicity. Caucasian and Asian populations are at higher risk of osteoporosis compared to African American and Hispanic populations, which are at lower risk.14
- Family history. Having a family member with osteoporosis may increase risk.
- Age. After age 40, bone mass declines due to bone break down exceeding bone formation.
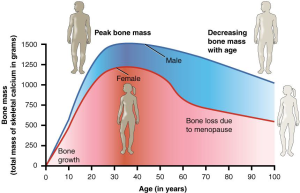
- Biological sex. Females, on average, have a lower peak bone mass compared with males (see Figure 16.1.8) and a much greater risk of developing osteoporosis, in part because of hormone levels.
- Hormones. Both estrogen and testosterone help to increase peak bone mass. Estrogen, a primary female reproductive hormone, stimulates bone building and reduces bone breakdown. When women go through menopause (usually around age 50), they experience a natural decline in estrogen levels, which accelerates bone loss and increases the risk of developing osteoporosis (see Figure 16.1.8).
Risk factors that can be controlled:
- Physical inactivity. Physical inactivity lowers peak bone mass, decreases BMD at all ages, and is linked to an increase in fracture risk, especially in the elderly. Regular exercise can help individuals achieve greater peak bone mass, prevents bone loss for women and men age 30 and older, and maintains strength and balance to help prevent falls later in life. The best activities for stimulating new bone are weight-bearing exercise like walking, hiking, climbing stairs, and resistance exercises like weight lifting.
- Nutrition. Ensuring adequate nutrition is a key component in maintaining bone health. Having low dietary intakes of calcium and vitamin D are strong risk factors for developing osteoporosis. Protein is also important during childhood and adolescence for proper bone development, and in older age to preserve bone mass.17
- Smoking. Smoking has long been correlated with a decrease in bone mass and an increased risk of osteoporosis and fractures.
- Alcohol intake. Alcohol intake may also affect bone health, although this seems to depend on the amount consumed. Light to moderate alcohol intake (two drinks or less per day) has been shown in some studies to be associated with an increase in bone density and a decreased risk of developing osteoporosis. However, excessive alcohol intake is associated with decreased bone density and increased fracture risk, although this may be due in part to other lifestyle factors, such as poor diet and less physical activity18
- Being underweight. Being underweight significantly increases the risk of developing osteoporosis, because people who are underweight often have a smaller frame size and a lower peak bone mass. The most striking relationship between being underweight and bone health is seen in people with anorexia. Anorexia is strongly correlated with low peak bone mass, and more than 50% of men and women who have this illness develop osteoporosis, often very early in life.
The changeable risk factors for osteoporosis provide ways for people to improve their bone health, even though some people may be genetically predisposed to the disease. Prevention of osteoporosis begins early in life since this is a critical time of bone growth. Eating a balanced diet that provides adequate amounts of calcium, phosphorus, magnesium, vitamin D, and protein is important for bone health throughout the life cycle. Participating in exercise such as walking, hiking, and weight lifting, and refraining from risky behaviors like smoking and excessive drinking are all behaviors that will help protect bones.
16.2 Blood
Blood is essential to life. It transports absorbed nutrients and oxygen to cells, removes metabolic waste products for excretion, and carries molecules, such as hormones, to allow for communication between organs. Blood is a connective tissue of the circulatory system, made up of four components forming a matrix:
- red blood cells (or erythrocytes), which transport oxygen to cells
- white blood cells (or leukocytes), which are part of the immune system and help destroy foreign invaders
- platelets, which are fragments of cells that circulate to assist in blood clotting
- plasma, which is the fluid portion of the blood and contains proteins that help transport nutrients (e.g., fat-soluble vitamins) and aid in blood clotting.
Maintaining healthy blood, including its continuous renewal, is essential to support its vital functions. Blood health is acutely sensitive to deficiencies in some vitamins and minerals, such as vitamin K and iron in particular.
Iron
Iron is a trace mineral. It is a necessary component of many proteins in the body and is responsible for numerous functions. Many of the proteins of the electron transport chain contain iron-sulfur clusters involved in the transfer of high energy electrons and ultimately ATP synthesis. Iron is also involved in numerous metabolic reactions that take place mainly in the liver and detoxify harmful substances. Moreover, iron is required for DNA synthesis and is important for brain development and function.
One of iron’s most well known functions is as an oxygen carrier. It is essential for oxygen transport because of its role in hemoglobin, a protein in red blood cells that transports oxygen to cells and gives red blood cells their color. Hemoglobin is composed of four globular peptides, each with an iron-containing heme complex in the center (Figure 16.2.1). In the center of each heme, lies iron. The iron in hemoglobin is what binds to oxygen in the capillaries of the lungs and transports it to cells where the oxygen is released. Iron is also an important part
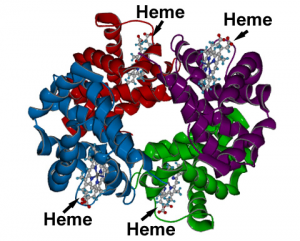
of myoglobin, a protein similar to hemoglobin but found in muscle tissue (myo = muscle).
If iron levels are low, hemoglobin is not synthesized in sufficient amounts, and the oxygen-carrying capacity of red blood cells is reduced, resulting in anemia. When iron levels are low in the diet the small intestine more efficiently absorbs iron in an attempt to compensate for the low dietary intake, but this process cannot make up for the excessive loss of iron that occurs with chronic low intake or blood loss. When blood cells are decommissioned for use, the body recycles the iron back to the bone marrow where red blood cells are made. The body stores some iron in the bone marrow, liver, spleen, and skeletal muscle. A relatively small amount of iron is excreted when cells lining the small intestine and skin cells die, and in blood loss, including menstrual bleeding. The lost iron must be replaced from dietary sources.
Table 16.2.1 Enhancers and Inhibitors of Iron Absorption
| Enhancer | Inhibitor |
| Meat | Phosphate |
| Fish | Calcium |
| Poultry | Tea |
| Seafood | Coffee |
| Stomach acid | High doses of minerals (antacids) |
| Vitamin C | High fiber |
| Phytates | |
| Oxalates | |
| Polyphenols |
Iron Sources and Requirements
There are two types of iron in foods: heme iron and non-heme iron. The bioavailability of iron is highly dependent on dietary sources. In animal-based foods about 40% of iron is bound to hemoglobin, and this heme iron is more bioavailable than non-heme iron. The other 60% of iron in animal based foods is non-heme, which is also the only iron source in plant-based foods. Bioavailability of iron is inhibited by several substances some plants contain (such as phytates and oxalates). However, eating iron-containing foods along with fruits and vegetables rich in vitamin C markedly increases iron absorption. Common enhancers and inhibitors of iron absorption are listed in Table 16.2.1.
Bioavailability is influenced by the dietary factors previously mentioned, as well as iron status. The body has no physiological mechanism to excrete iron; therefore, iron balance is tightly regulated by absorption.19 When iron stores are low, more dietary iron will be absorbed. The majority of the body’s iron needs are not met by dietary sources, but rather by recycling iron within the body. Ninety percent of daily iron needs are met by recycling iron released from the breakdown of aging cells, mostly red blood cells.
Table 16.2.2 Iron Content of Various Foods20
| Food | Serving | Iron (mg) | %DV |
| Breakfast cereal, enriched | 1 serving | 18 | 100 |
| Eastern oysters, cooked | 3 oz | 8 | 44 |
| White beans, canned | 1 c | 8 | 44 |
| Dark chocolate, 45-69% cacao | 3 oz | 7 | 39 |
| Beef liver, pan fried | 3 oz | 5 | 28 |
| Lentils, boiled and drained | ½ c | 3 | 17 |
| Spinach, boiled | ½ c | 3 | 17 |
| Tofu, firm | ½ c | 3 | 17 |
| Kidney beans, canned | ½ c | 2 | 11 |
| Atlantic sardines, canned in oil | 3 oz | 2 | 11 |
The RDA for iron is 8 mg/day for men and 18 mg/day for women. The RDA for vegetarians is 1.8 times higher (or 14 mg/day for men or 32 mg/day for women) than for people who eat meat. The UL for iron is set at 45 mg/day and applies to both food and supplements.20 Healthy adults are at little risk of iron overload from foods, but too much iron from supplements can result in gastric upset, constipation, nausea, vomiting, and abdominal pain.20 The body excretes little iron and therefore the potential for toxicity from supplements is a concern. Iron accumulation in certain tissues and organs can cause a host of health problems in children and adults including extreme fatigue, joint pain, and severe liver and heart toxicity. In children, death has occurred from ingesting as little as 200 mg of iron and therefore it is critical to keep iron supplements out of children’s reach.
Hemochromatosis is a hereditary disease resulting from a genetic mutation that leads to abnormal iron metabolism and an accumulation of iron in certain tissues such as the liver, pancreas, and heart. The signs and symptoms of hemochromatosis are similar to those of iron overload in tissues caused by a high intake of iron, but are often more severe and can include liver cirrhosis, cancer, heart disease, and impaired pancreatic function. It is usually diagnosed in middle age in men. In women it is usually not diagnosed until after menopause because, prior to that, monthly menstruation helps to mitigate the iron accumulation in tissues.
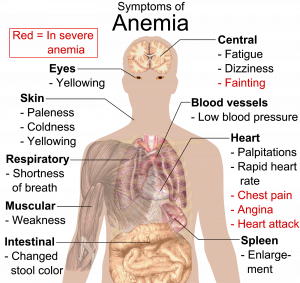
Iron Deficiency Anemia (IDA)
Iron deficiency anemia (IDA) is a condition that develops from having insufficient iron levels in the body resulting in fewer and smaller red blood cells containing lower amounts of hemoglobin. As a result, blood carries less oxygen from the lungs to cells. Regardless of the cause (be it from low dietary intake of iron or via excessive blood loss), IDA has the following signs and symptoms, which are linked to the essential functions of iron in energy metabolism and blood health:
- Fatigue
- Weakness
- Pale skin
- Shortness of breath
- Dizziness
- Swollen, sore tongue
- Abnormal heart rate
IDA is diagnosed from these characteristic signs and symptoms and confirmed with simple blood tests that count red blood cells and determine hemoglobin and iron content in blood. Anemia is most often treated with iron supplements and increased consumption of iron rich foods. Iron supplements have some adverse side effects including nausea, constipation, diarrhea, vomiting, and abdominal pain. Reducing the dose at first and then gradually increasing to the full dose often minimizes the side effects of iron supplements. Avoiding foods and beverages high in phytates and also tea (which contains tannic acid and polyphenols, both of which impair iron absorption) is important for people who have IDA. Eating a dietary source of vitamin C at the same time as iron containing foods improves absorption of non-heme iron in the gut. Additionally, unknown compounds that likely reside in muscle tissue of meat, poultry, and fish increase iron absorption from both heme and nonheme sources (see Table 16.2.1).
The WHO reports that iron deficiency is the most common nutritional deficiency worldwide, affecting nearly 30% of the world population.21 The main causes of iron deficiency worldwide are parasitic worm infections in the gut causing excessive blood loss, and malaria, a parasitic disease causing the destruction of red blood cells. In the developed world, iron deficiency is more the result of dietary insufficiency and/or excessive blood loss occurring during menstruation or childbirth.
At-Risk Populations
Infants, children, adolescents, and women are the populations most at risk worldwide for IDA by all causes.22 Infants, children, and teens require more iron because iron is essential for growth. In these populations, iron deficiency (and eventually IDA) can also cause the following signs and symptoms: poor growth or failure to thrive, and poor performance in school, as well as mental, motor, and behavioral disorders. Women who experience heavy menstrual bleeding or who are pregnant require more iron in the diet. One more high-risk group is the elderly. Both elderly men and women have a high incidence of anemia and the most common causes are dietary iron deficiency and chronic disease such as ulcer, inflammatory diseases, and cancer. Additionally, those who frequently donate blood, as well as people with cancer, heart failure, or gastrointestinal diseases such as Crohn’s, take excessive antacids for heartburn or who have suffered from taumatic blood loss need more iron in the diet.20
Preventing IDA
In young children IDA can cause significant motor, mental, and behavioral abnormalities that are long-lasting. In the US, the high incidence of IDA in infants and children was a major public-health problem prior to the early 1970s, but now the incidence has been greatly reduced. This achievement was accomplished by implementing the screening of infants for IDA in the health sector as a common practice, advocating the fortification of infant formulas and cereals with iron, and distributing them in supplemental food programs, such as Women, Infants, and Children (WIC). Breastfeeding, iron supplementation, and delaying the introduction of cow’s milk for at least the first year of life are also encouraged. These practices were implemented across the socioeconomic spectrum and by the 1980s, IDA in infants had significantly declined. Other solutions had to be introduced in young children, who no longer were fed breast milk or fortified formulas and were instead consuming cow’s milk. These recommendations for parents include: providing a diet rich in sources of iron and vitamin C, limiting cow’s milk consumption to less than 24 oz per day, and providing a multivitamin containing iron.
Vitamin K
Vitamin K refers to a group of fat-soluble vitamins that are similar in chemical structure. It was discovered in 1929.2 Vitamin K is critical for blood function acting as a coenzyme in blood coagulation (or blood clotting). Blood clotting proteins are continuously circulating in the blood. Upon injury to a blood vessel, platelets stick to the wound forming a plug. Without vitamin K, blood would not clot. Vitamin K is also required for maintaining bone health, as it modifies a protein which is involved in the bone remodeling process.

Vitamin K is found in the highest concentrations in green vegetables such as broccoli, cabbage, kale, parsley, spinach, and lettuce. Soybean and canola oil are also common sources of vitamin K in the US diet.23 Additionally, vitamin K can be synthesized via bacteria in the large intestine. The exact amount is unknown, but it is likely less than 10% of the recommended intake. Newborns have low vitamin K stores and it takes time for the sterile newborn gut to acquire the good bacteria it needs to produce vitamin K. Therefore, in the US it has become a routine practice to inject newborns with a single intramuscular dose of vitamin K. This practice has basically eliminated vitamin K dependent bleeding disorders in babies.
Table 16.2.3 Vitamin K Content of Various Foods23
| Food | Serving | Vitamin K (mcg) | %DV |
| Collard greens, boiled | ½ c | 530 | 442 |
| Turnip greens, boiled | ½ c | 426 | 355 |
| Spinach, raw | 1 c | 145 | 121 |
| Kale, raw | 1 c | 113 | 94 |
| Broccoli, boiled | ½ c | 110 | 92 |
| Soybeans, roasted | ½ c | 43 | 36 |
| Pumpkin, canned | ½ c | 20 | 17 |
| Pine nuts, dried | 1 oz | 15 | 13 |
| Blueberries | ½ c | 14 | 12 |
| Canola oil | 1 tbsp | 10 | 8 |
The AI for vitamin K is 120 mcg/day for men and 90 mcg/day for women. There is no UL currently for vitamin K because of its low potential for toxicity.23
Vitamin K deficiency is rare, but when it does occur can cause excessive bleeding. When there is damage to a blood vessel that results in bleeding, like a small tear, the body can stop this bleeding through a cascade of reactions. Without adequate vitamin K, blood does not clot properly, and this small bleed can turn into a larger problem, causing excessive bleeding or hemorrhaging. People at risk for vitamin K deficiency include those with liver or pancreatic disease, or malabsoprtion conditions such as celiac disease. Signs and symptoms of deficiency include nosebleeds, easy bruising, broken blood vessels, bleeding gums, and heavy menstrual bleeding in women. Anticoagulant drugs, such as warfarin, are prescribed for those at high risk of developing blood clots that could lead to a stroke or heart attack. These drugs act in opposition to vitamin K and therefore, those who are taking such medicine may need to limit their vitamin K intake. Some newer drugs are now on the market that do not require modification of dietary vitamin K.
Additional Micronutrients that Contribute to Blood Health
While iron and vitamin K are particularly associated with supporting blood health, several other micronutrients are also involved. Vitamin B6, niacin, riboflavin, pantothenic acid, copper, zinc and sulfur are all required for the production and function of red blood cells. Vitamins B6, B12, folate, zinc, and iron play important roles red blood cell division. And while IDA is the most common form of anemia, deficiencies of folate, B6, and B12 can also cause types of anemia. Vitamin E, niacin, riboflavin, zinc, copper, and selenium all work to protect red blood cell membranes from oxidative damage. While vitamin K is particularly known for blood clotting, it requires the help of calcium for these clots to function.13
16.3 Antioxidants
Before you can understand what an antioxidant is, it is important to have an understanding of oxidants. In chemistry, oxidation occurs when a molecule loses an electron. If another molecule picks up the lost electron, we say that this molecule with an added electron is reduced. Although this sounds like the opposite of addition, because electrons are negatively charged, the addition of a negative “reduces” the charge of the molecule. This oxidation-reduction system occurs in many instances in the body and in the environment.
Electrons prefer to be partnered together in molecules, so when an electron is lost and oxidation occurs, one electron is unpaired. A molecule with an unpaired electron is called a free radical. This molecule becomes unstable and highly reactive because it actively seeks an electron to partner with for stability. As one molecule steals from another, they create additional new free radicals and damage other important molecules along the way, similar to how one falling domino can bring down countless more. These free radicals are called reactive oxidative species (ROS). Sometimes there are so many ROS formed that there are not enough extra electrons to pair with. When the formation of free radicals exceeds the body’s ability to provide electron relief, the body comes under oxidative stress, and the body cells and tissues become damaged.
Free radicals can be generated by a variety of sources that can be endogenous (coming from within the body) or exogenous (coming from outside the body). Endogenous sources include the body’s mitochondria where most of the body’s energy production occurs. High-intensity, long duration exercise like distance running, for example, increases the production of free radicals. Exogenous sources include ultraviolet light, ozone, radiation, heavy metals, smoking, air pollution, and asbestos.24 Accumulation of free radicals can cause inflammation. Free radical-induced damage, when left unrepaired, destroys lipids, proteins, RNA, and DNA, and can contribute to disease. Oxidative stress has been implicated as a contributing factor to cancer, atherosclerosis, arthritis, diabetes, kidney disease, Alzheimer’s disease, Parkinson’s disease, schizophrenia, bipolar disorder, emphysema, and cataracts.25
Not all free radicals are bad. While our bodies have acquired multiple defenses against them, we also use free radicals to support its functions. For example, the immune system uses the cell-damaging properties of free radicals to kill pathogens. First, immune cells engulf an invader (such as a bacterium), then they expose it to free radicals such as hydrogen peroxide, which destroys its membrane. The invader is thus neutralized. Scientific studies also suggest free radicals may aid with tissue repair when you get cut, help produce thyroid hormone, help dilate blood vessels, and act as chemical signaling molecules. However, we must attempt to maintain balance between the number of free radicals and our ability to neutralize them to avoid oxidative stress.
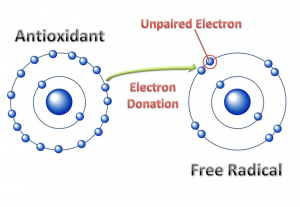
Antioxidants
Antioxidants are molecules that can combat free radicals and oxidative stress. They donate one of their electrons to a free radical to generate a stable compound (Figure 16.3.1). Many micronutrients (and other compounds) serve as antioxidants (Table 16.3.1), and consuming a varied diet, rich in colorful plants is highly recommended to provide these critical nutrients that are free radical fighters.
Table 16.3.1 Some Antioxidants Obtained from the Diet and Their Related Functions
| Antioxidant | Functions Attributed to Antioxidant Capacity |
| Beta-carotene | Protects cellular membranes, prevents glutathione depletion, maintains free radical detoxifying enzyme systems, reduces inflammation |
| Vitamin C | Protects DNA, RNA, proteins, and lipids, aids in regenerating vitamin E |
| Vitamin E | Protects cellular membranes, prevents glutathione depletion |
| Selenium | Neutralizes free radicals, aids in regeneration of glutathione and vitamin C |
| Carotenoids | Free radical scavengers |
| Lipoic acid | Free radical scavengers, aids in regeneration of vitamins C and E |
| Phenolic acids | Free radical scavengers, protect cellular membranes
|
Antioxidant Supplementation?
The market is flooded with advertisements for “super antioxidant” supplements teeming with molecules that claim to block free radical production, stimulate the immune system, prevent cancer, and reduce the signs of aging. Based on the antioxidant supplement industry’s success, the general public appears to believe these claims. However, such claims are not backed by scientific evidence; rather there is evidence suggesting antioxidant supplements can actually cause harm. For example, high doses of beta-carotene supplements was found to increase the risk of lung cancer in smokers, and high doses of vitamin E supplements was found to increase the risk of prostate cancer in men. Antioxidant supplements may also interact with other medications, further emphasizing the importance of talking with your doctor before taking any type of supplement.26 (More on dietary supplements in Chapter 17). There is, however, evidence supporting the consumption of antioxidant-rich foods, primarily vegetables and fruits, as a method of reducing risk of chronic disease. Unfortunately there is no “miracle cure.” No pill or supplement alone can provide the same benefits of a varied and healthful diet.
Let’s take a closer look at some of the most important dietary antioxidants: vitamin E, vitamin C, and selenium.
Vitamin E
Vitamin E occurs in eight chemical forms, but only one of these, alpha-tocopherol, appears to meet human requirements. It was discovered in 1922.2 Because vitamin E is fat-soluble, its antioxidant capacity is especially important to lipids, including those in cell membranes and lipoproteins. For example, free radicals can oxidize LDL cholesterol and it is this damaged LDL that lodges in blood vessels and forms the fatty plaques characteristic of atherosclerosis, increasing the risk of heart attack, stroke, and other complications of cardiovascular disease. In addition to its antioxidant functions, vitamin E plays a role in the immune system, regulation of gene expression, and cell signaling. It also enhances the dilation of blood vessels and inhibits blood clot formation.
Once alpha-tocopherol interacts with a free radical it is no longer capable of acting as an antioxidant unless it is enzymatically regenerated. Vitamin C helps to regenerate some, but the remainder is eliminated from the body. Therefore, to maintain vitamin E levels, you need to regularly consume it as part of your diet.
Vitamin E Sources and Requirements
Vitamin E is found in many foods, especially those higher in fat, such as nuts and oils. Some spices, such as paprika and red chili pepper, and herbs, such as oregano, basil, cumin, and thyme, also contain vitamin E. But keep in mind spices and herbs are commonly used in small amounts in cooking and therefore are a lesser source of dietary vitamin E. Surveys of Americans’ diets often find that they provide less than the RDA for vitamin E. However, these studies may underestimate the amount of vitamin E in the diet because they don’t fully account for vegetable oils in the diet, as these are rich sources of vitamin E. Vitamin E can be destroyed at high temperatures, especially when reheated repeatedly, so oils used in deep frying are not good sources of the vitamin.
Table 16.3.2 Vitamin E Content of Various Foods27
| Food | Serving | Vitamin E (mg) | %DV |
| Sunflower seeds, dry roasted | 1 oz | 7.4 | 49 |
| Almonds, dry roasted | 1 oz | 6.8 | 45 |
| Sunflower oil | 1 tbsp | 5.6 | 37 |
| Hazelnuts, dry roasted | 1 oz | 4.3 | 29 |
| Peanut butter | 2 tbsp | 2.9 | 19 |
| Spinach, boiled | ½ c | 1.9 | 13 |
| Broccoli, boiled | ½ c | 1.2 | 8 |
| Kiwifruit | 1 medium | 1.1 | 7 |
| Tomato | 1 medium | 0.7 | 5 |
| Mango, sliced | ½ c | 0.7 | 5 |
The AI for vitamin E is 15 mg mg/day for men and women. The UL for vitamin E is 1,000 mg/day and applies only to supplemental intake. As mentioned, high dose vitamin E supplements increased risk of cancer in men and supplementation of alpha-tocopherol in animals has been shown to disrupt blood coagulation, causing hemorrhage, stroke and death.27
Vitamin E deficiency is rare and overt deficiency symptoms have not been seen in healthy people. People with malabsorption disorders, such as Crohn’s disease or cystic fibrosis, and babies born prematurely, are at higher risk for vitamin E deficiency. Symptoms include nerve and muscle damage, vision problems, and a weakened immune system.27
Vitamin C
Vitamin C, also called ascorbic acid, is a water-soluble micronutrient essential for humans, though most other mammals can readily synthesize it. It was first discovered in 1912.2 Vitamin C’s ability to easily donate electrons makes it a highly effective antioxidant. Since it is water-soluble, it acts both inside and outside cells. Vitamin C also plays a vital role in regenerating vitamin E after it has acted as an antioxidant, allowing it to be recycled and used again. It can also very effectively regenerate itself and can exist in the body functioning as an antioxidant for many weeks.
In addition to its role as an antioxidant, vitamin C is a required part of several enzymes such as signaling molecules in the brain, some hormones, and amino acids. It is essential for the synthesis and maintenance of collagen, which is the most abundant protein in the body and used for different functions such as the structure for ligaments, tendons, blood vessels, skin and scars that bind wounds together. Vitamin C acts as the glue that holds the collagen fibers together. Vitamin C also improves the absorption of dietary iron.
Vitamin C does have several roles in the immune system, and often people will increase vitamin C intake either from diet or supplements when they have a cold. Many others routinely consume vitamin C in an effort to prevent colds. Contrary to this popular practice, there is limited evidence that this works. A review of more than 50 years of studies concluded that taking vitamin C routinely does not prevent colds in most people, but it may slightly reduce cold severity and duration. Moreover, taking megadoses (up to 4000 mg/day) at the onset of a cold provided no benefits.28 The body’s vitamin C status is tightly controlled to maintain steady tissue and plasma concentrations. This means that if you consume high doses of vitamin C, you’ll absorb less from the intestine and excrete more in urine to prevent excessive concentrations in the body. In fact, people taking megadoses of vitamin C will often see their urine color become more saturated.
Vitamin C Sources and Requirements
Citrus fruits are great sources of vitamin C as are many other fruits and vegetables. It is not found in significant amounts in animal-based foods. Because vitamin C is water-soluble, it leeches away from foods considerably during cooking, freezing, thawing, and canning. Therefore to maximize vitamin C intake from foods, you should eat fruits and vegetables raw or lightly steamed.
Table 16.3.3 Vitamin C Content of Various Foods29
| Food | Serving | Vitamin C (mg) | %DV |
| Sweet red peppers, raw | ½ c | 95 | 106 |
| Orange juice | ¾ c | 93 | 103 |
| Orange | 1 medium | 70 | 78 |
| Broccoli, cooked | ½ c | 51 | 57 |
| Strawberries | 1 c | 49 | 54 |
| Brussels sprouts, cooked | ½ c | 48 | 53 |
| Grapefruit | ½ medium | 39 | 43 |
| Cantaloupe | ½ c | 29 | 32 |
| Tomato | 1 medium | 17 | 19 |
| Potato, baked | 1 medium | 17 | 19 |
The RDA for vitamin C is 90 mg/day for men and 75 mg/day for women. Individuals who smoke require an additional 35 mg/day. The UL (for both food and supplement use) is set at 2000 mg/day although vitamin C has low toxicity and is not believed to cause serious adverse effects at high intakes. The most common complaints relate to gastrointestinal disturbances such as diarrhea, nausea, and abdominal cramps.29
The classic condition associated with vitamin C deficiency is scurvy. Signs and symptoms include skin disorders, bleeding gums, painful joints, weakness, depression, and increased susceptibility to infections.
Scurvy
Between the 15th and 18th centuries, scurvy killed more than two million sailors including many on the ships captained by Christopher Columbus. This was the age of great sailing voyages across the Atlantic, Pacific, and Indian Oceans and sailors could be at sea for more than three months at a time. They would suffer greatly from many vitamin deficiencies because the dried and preserved foods they consumed did not contain adequate micronutrients, but the worst was scurvy. The symptoms of scurvy were severe. It would begin with small blisters that would develop into painful, large, bleeding sores. Gums would become black, teeth would fall out. Without vitamin C the body can no longer make collagen, so previously broken bones would rebreak, and old wounds would open up. Arteries would decay so a person would bleed internally. The structure of the brain would deteriorate causing personality changes, vivid dreams, hallucinations, and seizures.30 Sailors who ate the ship’s rats were spared because rats make their own vitamin C. In 1622, Sir Richard Hawkins wrote that “sower (sour) lemons and oranges” helped, but it wasn’t until over 100 years later in 1747 that Englishman James Link conducted the first controlled medical experiment on a ship showing that citrus fruits cured scurvy.31 This was more than 150 years before vitamin C was “discovered.”
Selenium
Selenium is an essential trace mineral. Approximately 25 known proteins require selenium to function. Some are enzymes involved in detoxifying free radicals and include glutathione peroxidases and thioredoxin reductase. As an integral functioning part of these enzymes, selenium aids in the regeneration of glutathione and oxidized vitamin C. Selenium as part of glutathione peroxidase also protects lipids from free radicals, and, in doing so, spares vitamin E. This is just one example of how antioxidants work together to protect the body against free radical induced damage.
Selenium also acts as a cofactor of enzymes that convert the inactive thyroid hormone to the active form in cells, and therefore low levels can cause similar signs and symptoms as iodine deficiency. Other functions of selenium containing proteins include protecting endothelial cells that line tissues and mediating inflammatory and immune system responses.
Seafoods and meat have some of the highest selenium content. Plants do not require selenium, so the content in fruits and vegetables is usually low. However, selenium levels are higher in plants grown in selenium-rich soils. The selenium content of soil used to grow animal feed can also affect the selenium content of animal products.
Table 16.3.4 Selenium Content of Various Foods32
| Food | Serving | Selenium (mcg) | %DV |
| Brazil nuts (6-8 nuts) | 1 oz | 544 | 989 |
| Yellowfin tuna, cooked | 3 oz | 92 | 167 |
| Halibut, cooked | 3 oz | 47 | 85 |
| Ham, roasted | 3 oz | 42 | 76 |
| Turkey, boneless, roasted | 3 oz | 31 | 56 |
| Cottage cheese (1%) | 1 c | 20 | 36 |
| Egg, hard boiled | 1 large | 15 | 27 |
| Bread, whole wheat | 1 slice | 13 | 24 |
| Vegetarian baked beans, canned | 1 c | 13 | 24 |
| Milk (1%) | 1 c | 8 | 15 |
The RDA for selenium is 55 mcg/day for men and women. Selenium at doses several thousand times the RDA can cause acute toxicity, and when ingested in gram quantities can be fatal. The UL for selenium is set for 400 mcg/day. Chronic intake of high levels of selenium can lead to selenosis, characterized by hair loss, nail brittleness, nausea, diarrhea, and lesions of the skin and nervous system.
Selenium deficiency is very rare in the US and most other developed countries. Worldwide, people with a primarily vegetarian diet in areas with low soil selenium levels, including parts of China and Europe, may be at risk for selenium deficiency.32
Additional Micronutrients that Function in Antioxidant Processes
In addition to vitamin C, E, and selenium, other trace minerals copper, zinc, and manganese function as components of enzymes involved in antioxidant processes. Carotenoids, such as beta-carotene (a precursor to vitamin A), can also serve as antioxidants.13 These will be discussed in the next section.
16.4 Vision
Vision is one of our five basic senses, and unless you’ve experienced vision loss, it’s hard to imagine life without sight. Vitamin A plays a key role in vision and eye health. In this section, we’ll cover the functions, food sources, and signs of toxicity and deficiency for vitamin A and carotenoids such as beta-carotene, which can be converted to vitamin A.
Vitamin A
Vitamin A, a generic term for a group of similar fat-soluble compounds called retinoids, was discovered in 1915.2 Retinol is the form of vitamin A found in animal-derived foods. In the body it is converted to biologically active forms, retinal and retinoic acid. These are called preformed or active vitamin A. This differentiates from carotenoid compounds, which are brightly colored yellow, orange, and red pigments synthesized by plants. These are called provitamin A, because they can be converted to vitamin A in the body. Over 600 carotenoids have been identified, with beta-carotene being one of the most well known. Approximately 10% of carotenoids can be converted in the body to retinoids. Enzymes in the intestine and liver can cleave the beta-carotene molecule in half, creating two new molecules of vitamin A.
As with other fat-soluble vitamins, vitamin A is packaged into chylomicrons in the small intestine and absorbed into the lymphatic system before being transported to the liver. The liver stores and exports vitamin A as needed; it is released into the blood bound to a retinol-binding protein, which transports it to cells. Beta-carotene can be converted into vitamin A in the intestine, or it can be absorbed intact, packaged in chylomicrons, and then transported around the body. As was discussed in the previous section, beta-carotene and other carotenoids that aren’t converted to vitamin A can also act as powerful antioxidants protecting cellular membranes, helping maintain glutathione levels, and influencing the amount and activity of enzymes that detoxify free radicals.
The retinoids are aptly named, as their most notable function is in the retina of the eye. Retinol that is circulating in the blood is taken up by cells in the retina, where it is converted to retinal and is used as part of the pigment rhodopsin. Rhodopsin is especially important to our ability to see in low light conditions. When light hits rhodopsin in the eye, a nerve signal is sent to the brain, allowing us to detect that light. A person that is deficient in vitamin A has less rhodopsin pigment in the eye and is therefore less able to detect low level light. This makes it more difficult to see at night, a condition referred to as night blindness, and this is one of the first and most definitive signs of vitamin A deficiency.
Vitamin A is also required for normal cellular differentiation, the process by which cells change from stem cells to more specialized cells with specific structure and function. Cellular differentiation is important in every tissue of the body, but if there is a shortage of vitamin A, the eye is one of the first areas to be impacted. Specialized cells in the lining of the eyes produce mucus and tears, which keep eyes moist and lubricated. When the mucus secreting cells die, they need to be replaced with new cells. If the body is deficient in vitamin A, those new cells don’t differentiate normally, resulting in dry eyes, a condition called xerophthalmia. Instead of producing mucus, these dysfunctional cells produce a protein called keratin. Keratin is a hard, structural protein that is found in nails, hair, and the outer layer of skin, and you can imagine the problems it causes when it accumulates in the eye. Instead of a moist, well-lubricated eye, keratin makes the eye hard and dry, resulting in clouded vision.
Caught early, these eye conditions may be reversed with adequate vitamin A intake. However, severe vitamin A deficiency over time can cause complete vision loss. In fact, vitamin A deficiency is the number one cause of preventable blindness worldwide, especially in children. According to the WHO, an estimated 250,000 to 500,000 children lose their sight each year due to vitamin A deficiency, and half of them die within a year of developing blindness, likely due to infection.33 It is caused by malnutrition related to consumption of inadequate diets predominantly based on staple grains and lacking in animal products, fruits, vegetables, and fat, which increases absorption of vitamin A.
Vitamin A’s role in cellular differentiation also makes it critical to cells around the body involved in normal growth, development, reproduction, and immune function. All of these processes require cells to develop in specific ways at specific times, and vitamin A helps to orchestrate these processes. For example, embryonic development requires stem cells to differentiate into specific types of cells to form new organs, and timing is critical. Vitamin A also helps the immune system produce different types of immune cells, and without adequate vitamin A, a person is more susceptible to infections.
Consuming excessive amounts of vitamin A during pregnancy, however, can also cause birth defects, so pregnant people should pay close attention to vitamin A contained in supplements. In addition, some synthetic forms of vitamin A (Retin-A and Accutane, for example) are used as acne treatments and should never be used during pregnancy. Unlike preformed vitamin A, beta-carotene and other carotenoids do not seem to cause birth defects or other major toxicity effects in high doses. This is because the body doesn’t convert beta-carotene to vitamin A if it already has excessive amounts of vitamin A. Because it doesn’t cause toxicity, beta-carotene is usually used as the source of vitamin A in prenatal multivitamin supplements.
Vitamin A Sources and Requirements
Preformed vitamin A is found only in animal-derived foods with liver being the richest source because that’s where it is stored. Carotenoids are brightly colored pigments, so vibrant color is a good indicator of their presence. Top sources include orange and yellow vegetables such as carrots, sweet potatoes, and pumpkin (beta-carotene is a bright orange pigment), bell peppers, many fruits, leafy green vegetables, and some vegetable oils. Some carotenoids can also be found in animal-derived foods. For example, the yellow color of egg yolk and butter comes from carotenoids absorbed from the diets of the hens and cows, respectively. Beta-carotene that isn’t converted to vitamin A is absorbed intact in the intestine. When high levels of beta-carotene are consumed in the diet, it can have the unusual effect of making a person’s skin appear to be yellow or orange. The color change doesn’t seem to be harmful, and normal skin tone returns once the person stops consuming so much beta-carotene.
Table 16.4.1 Vitamin A Content of Various Foods35
| Food | Serving | Vitamin A (mcg RAE) | %DV |
| Beef liver, pan fried | 3 oz | 6,582 | 731 |
| Sweet potato, baked | 1 whole | 1,403 | 156 |
| Spinach, boiled | ½ c | 573 | 64 |
| Carrots, raw | ½ c | 459 | 51 |
| Ricotta cheese, part-skim | 1 c | 263 | 29 |
| Atlantic herring, pickled | 3 oz | 219 | 24 |
| Milk, skim | 1 c | 149 | 17 |
| Cantaloupe, raw | ½ c | 135 | 15 |
| Sweet red peppers, raw | ½ c | 117 | 13 |
| Egg, hard boiled | 1 large | 75 | 8 |
The RDA for vitamin A is 900 mcg RAE/day for men and 700 mcg RAE/day for women. RAE refers to retinol activity requirements as it takes into account its many different available forms. Therefore, 1 mcg of retinol is equivalent to 12 mcg of beta-carotene. The UL for vitamin A is 3,000 mcg/day and this applies to preformed vitamin A and supplemental intake. Vitamin A toxicity causes dry, itchy skin, loss of appetite, dizziness, nausea, swelling of the brain, and joint pain. In the most severe cases, it can cause liver damage, coma, and death.35 Vitamin A toxicity is almost always caused by using excessive supplements for substantial periods. Vitamin A supplements have been found to increase the risk of lung cancer in people who are at high risk for the disease (i.e., smokers, ex-smokers, workers exposed to asbestos). The Beta-Carotene and Retinol Efficacy Trial (CARET) involving over 18,000 participants who were at high risk for lung cancer found that people who took supplements containing very high doses of vitamin A and beta-carotene had a 28% higher incidence of lung cancer midway through the study, which was consequently stopped.36 These high doses would not be found in a normal diet, although vitamin A toxicity has been observed in Arctic explorers who ate large amounts of bear and seal liver.35
Other Micronutrients that Contribute to Eye Health and Vision
Recent research suggests that vitamin C is required for nerve cells in the eyes to function properly. The eyes have a particularly high metabolic rate and therefore need added antioxidant protection. Because of their roles as an antioxidants, both vitamin C and vitamin E are thought to help protect eye cells from premature breakdown and prevent eye diseases cataracts and macular degeneration (a leading cause of blindness).37,38 Zinc is also required for maintaining the health of the retina.38
16.5 Immunity
The immune system is comprised of several types of white blood cells that circulate in the blood and lymph. Their jobs are to see, recruit, attack, and destroy foreign invaders, such as bacteria and viruses. Other less realized components of the immune system are the skin (which acts as a barrier), mucus (which traps and entangles microorganisms), and the bacteria of the gut microbiome in the large intestine (which prevents colonization of bad bacteria). Immune system functions are completely dependent on dietary nutrients. In fact, malnutrition is the leading cause of immune system deficiency worldwide. When immune system functions are inadequate there is a marked increase in the chance of getting an infection.
With the recent COVID-19 pandemic, there has been has been an increased focus on micronutrient support for improved immunity. It’s true that together, nearly every vitamin and mineral plays a role in the proper functioning of our immune systems. These nutrients play a role in immune cell division and development, regulation of immune response, process of attacking pathogens, and protecting immune cells from free radicals that it produces to kill pathogens.13
Research suggests that the strongest evidence for immune support are vitamins C and D and zinc. In addition to its antioxidant effects, vitamin C stimulates the production, differentiation, and proliferation of certain white blood cells (e.g., T cells, lymphocytes) and contributes to increased generation of antibodies, among several other activities.39 But as stated previously, there is limited evidence that taking vitamin C prevents or reduces duration of the common cold.28 Deficiency of vitamin D has been linked to increased risk of several autoimmune diseases (e.g., multiple sclerosis, lupus, rheumatoid arthritis). Vitamin D, as calcitriol, helps regulate antimicrobial proteins and macrophages that can kill pathogens, and can help reduce pro-inflammatory cytokines.39 Zinc deficiency results in suppression of the immune system’s barrier functions by damaging skin cells; it is also associated with a decrease in the number of circulating white blood cells.
Does this mean you should consume these micronutrients in supplemental form to ensure you are meeting your needs? Not necessarily. Studies have found conflicting evidence of the benefit of some micronutrient supplements, and as stated previously, some have shown increased risk of cancers, especially at megadose levels.27,36 Your best chances of improving or maintaining your immune system is to eat a wide variety of fruits, vegetables, lean proteins, and whole grains in order to consume the RDA/AI for these important micronutrients. And make sure to exercise regularly and don’t smoke.
Key Takeaways
- Bones are living tissues that are in a constant state of breaking down and rebuilding. They also store several minerals including calcium, phosphorus, and magnesium.
- Calcium is the most abundant mineral in the body as the structural portion of bones and teeth. It also plays roles in nerve transmission, muscle contraction, blood clotting. Blood calcium levels are closely regulated by PTH, calcitriol, and calcitonin. Good dietary sources of calcium are dairy products, legumes, leafy greens.
- Vitamin D, a fat-soluble vitamin, is also called the “sunshine vitamin” as we can synthesize it via UV sunlight. It increases absorption of calcium and phosphorus, playing a critical role in the bone mineralization and blood calcium homeostasis. Vitamin D is also important for gene expression, cell differentiation, the immune system, and blood pressure. Rickets in children and osteomalacia in adults can result from vitamin D deficiency.
- Phosphorus, the second most abundant mineral in the body is a primary mineral component of bone. In addition, it is a component of cell membranes, RNA, DNA and is needed for energy production.
- Magnesium is also stored primarily in the bone as well as being required for more than 300 enzymatic reactions. Many Americans do not meet the RDA for magnesium.
- Fluoride, a trace mineral primarily combats tooth decay. It also plays a role in bone development and maintenance. The majority of fluoride in a person’s diet is from fluoridated water.
- Excessive bone loss can lead to the development of osteopenia and eventually osteoporosis. Osteoporosis is often considered a silent disease that doesn’t manifest until a fracture occurs.
- Iron is a trace mineral that is most well known as an oxygen carrier in the blood. It also functions as part of DNA and ATP synthesis, detoxification in the liver, and brain development and function. Iron is found in food as heme or non-heme iron. There are several other dietary components that can enhance or inhibit iron’s bioavailability. Women require higher amounts of iron daily.
- Iron deficiency anemia (IDA) is the most common nutrient deficiency in the world. Symptoms include fatigue, weakness, and pale skin as a result of the having fewer and smaller red blood cells carrying lower amounts of hemoglobin.
- Vitamin K is a coenzyme that participates in the modification of proteins that act in bone tissues and promotes normal blood clotting.
- Free radicals, unstable molecules with unpaired electrons, can steal electrons from other compounds such as RNA and DNA, resulting in damage. However, the body can sometimes use some free radicals for beneficial functions.
- Oxidative stress is when there is an imbalance between free radical production and repair systems. Antioxidants can combat free radicals and oxidative stress. Consuming antioxidant-rich foods (brightly colored plants) is one of the best ways to reduce your risk of disease. Vitamins E and C, beta-carotene, and selenium are considered antioxidants.
- Vitamin E, in the form of alpha-tocopheral is a fat-soluble vitamin that can protect cell membranes and lipoproteins from free radical damage. While important to consume in the form of food, studies have found that supplemental use has increased risk of cancer in some populations.
- Beyond its antioxidant capabilities, vitamin C is an important part of several enzymes, and is essential for the synthesis and maintenance of collagen. As a water-soluble vitamin, if one has already met their vitamin C needs, excess amounts in the body will be excreted in urine.
- Nearly every vitamin and mineral plays a role in the proper functioning of our immune system. Malnutrition is the leading cause of immune system deficiency worldwide. Your best chances of improving or maintaining your immune system is to eat a wide variety of fruits, vegetables, lean proteins, and whole grains to consume the RDA/AI for vital micronutrients.
Portions of this chapter were taken from OER Sources listed below:
Callahan, A., Leonard, H., Powell, T. (2020). Nutrition: Science and Everyday Application. https://openoregon.pressbooks.pub/nutritionscience/
Jellum, L., Hitzeman, J., Krauss, M., Henderson, S., Harnden, T., Elsberry, C., & Ford, G. (2018). Principles of Nutrition Textbook, Second Edition. Nursing and Health Sciences Open Textbooks. 5. https://oer.galileo.usg.edu/health-textbooks/5
Tharalson, J. (2019). Nutri300:Nutrition. https://med.libretexts.org/Courses/Sacremento_City_College/SSC%3A_Nutri_300_(Tharalson)
Titchenal, A., Calabrese, A., Gibby, C., Revilla, M.K.F., & Meinke, W. (2018). Human Nutrition. University of Hawai’i at Manoa Food Science and Human Nutrition Program Open Textbook. https://pressbooks.oer.hawaii.edu
References
- Office of Dietary Supplements. (2020, March 26). Calcium. National Institute of Health. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Price, C. (2015). Vitamania: How vitamins revolutionized the way we think about food. Penguin Books.
- Higdon, J., Drake, V., & Delage, B. (2014, April 23). Vitamin D. Linus Pauling Institute. https://lpi.oregonstate.edu/mic/vitamins/vitamin-D#deficiency
- Office of Dietary Supplements. (2020, March 24). Vitamin D. National Institute of Health. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Lemoine, A., Giabicani, E., Lockhart, V., Grimprel, E., & Tounian, P. (2020). Case report of nutritional rickets in an infant following a vegan diet. Archives De Pediatrie: Organe Officiel De La Societe Francaise De Pediatrie. https://doi.org/10.1016/j.arcped.2020.03.008
- Higdon, J., Drake, V., & Delage, B. (2014, April 23). Phosphorus. Linus Pauling Institute. https://lpi.oregonstate.edu/mic/minerals/phosphorus
- Office of Dietary Supplements. (2020, June 4). Phosphorus. National Institute of Health. https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/
- Office of Dietary Supplements. (2020, March 24). Magnesium. National Institute of Health. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- National Institute of Dental and Craniofacial Research. (2018, July). Dental caries (tooth decay). National Institutes of Health. https://www.nidcr.nih.gov/research/data-statistics/dental-caries.
- Centers for Disease Control and Prevention. (2020, January 15). Community water fluoridation. https://www.cdc.gov/fluoridation/index.html
- Centers for Disease Control and Prevention. (2019, March 8). Fluorosis. https://www.cdc.gov/fluoridation/faqs/dental_fluorosis/index.htm
- Institute of Medicine. (1997, January 1). Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. The National Academies Press. http://www.iom.edu/Reports/1997/Dietary-Reference-Intakes-for-Calcium-Phosphorus-Magnesium-Vitamin-D-and-Fluoride.aspx.
- Pope, J., Nizielski, S., & McCook, A. (2016). Nutrition for a changing world. (1st ed.). W. H. Freeman.
- National Institute of Arthritis and Musculoskeletal and Skin Diseases. (2019). Osteoporosis overview. https://www.bones.nih.gov/health-info/bone/osteoporosis/overview
- US Preventative Services Task Force (2018, June 26). Osteoporosis to prevent fractures: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/osteoporosis-screening
- National Institute of Arthritis and Musculoskeletal and Skin Diseases. (2018). Osteoporosis: Peak bone mass in women. https://www.bones.nih.gov/health-info/bone/osteoporosis/bone-mass
- International Osteoporosis Foundation. (n.d.). Nutrition. Retrieved April 28, 2020 from https://www.iofbonehealth.org/nutrition
- Gaddini, G. W., Turner, R. T., Grant, K. A., & Iwaniec, U. T. (2016). Alcohol: A simple nutrient with complex actions on bone in the adult skeleton. Alcoholism, Clinical and Experimental Research, 40(4), 657–671. https://doi.org/10.1111/acer.13000
- Hurrrell, R., & Egli, I. (2010). Iron bioavailability and dietary reference values. The American Journal of Clinical Nutrition, 91(5), 1461S–1467S. https://doi.org/10.3945/ajcn.2010.28674F
- Office of Dietary Supplements. (2020, February 28). Iron. National Institute of Health. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
- World Health Organization. (n.d.). Micronutrient deficiencies. https://www.who.int/nutrition/topics/ida/en/
- Powers, J. M., & Buchanan, G. R. (2019). Disorders of iron metabolism: New diagnostic and treatment approaches to iron deficiency. Hematology/Oncology Clinics of North America, 33(3),393-408. doi: 10.1016/j.hoc.2019.01.006
- Office of Dietary Supplements. (2020, June 3). Vitamin K. National Institute of Health. https://ods.od.nih.gov/factsheets/vitaminK-HealthProfessional/
- Lobo, V., Patil, A., Phatak, A., & Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews, 4(8), 118-126. https://doi.org/10.4103/0973-7847.70902
- Phaniendra, A., Jestadi, D. B., & Periyasamy, L. (2015). Free radicals: Properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry, 30(1), 11-26. https://doi.org/10.1007/s12291-014-0446-0
- National Center for Complementary and Integrative Health. (n.d.). Antioxidants: In Depth. Retrieved April 2, 2020, from https://www.nccih.nih.gov/health/antioxidants-in-depth
- Office of Dietary Supplements. (2020, February 28). Vitamin E. National Institute of Health. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Douglas, R.M., Hemilä, H., Chalker, E., D’Souza R. R.D., Treacy, B., & Douglas, B. (2004). Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews 4. https://doi.org/10.1002/14651858.CD000980.pub2
- Office of Dietary Supplements. (2020, February 27). Vitamin C. National Institute of Health. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Worrall, S. (2017, January 15). A nightmare disease haunted ships during the age of discovery. National Geographic. https://www.nationalgeographic.com/news/2017/01/scurvy-disease-discovery-jonathan-lamb/#close
- White, M. (2016, October 4). James Lind: The man who helped to cure scurvy with lemons. BBC.com. https://www.bbc.com/news/uk-england-37320399
- Office of Dietary Supplements. (2020, March 11). Selenium. National Institute of Health. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- World Health Organization. (n.d.). Vitamin A deficiency. Nutrition Landscape Information System. https://www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency
- Sommer, A. (2008). Vitamin a deficiency and clinical disease: An historical overview. The Journal of Nutrition, 138(10), 1835–1839. https://doi.org/10.1093/jn/138.10.1835
- Office of Dietary Supplements. (2020, February 14). Vitamin A. National Institute of Health. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Goodman, G. E., Thornquist, M. D., Balmes, J., Cullen, M. R., Meyskens Jr, F. L., Omenn, G. S., Valanis, B., & Williams, J. H. (2004). The beta-carotene and retinol efficacy trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. Journal of the National Cancer Institute, 96(23), 1743-1750. https://doi.org/10.1093/jnci/djh320
- Calero, C. I., Vickers, E., Moraga Cid, G., Aguayo, L. G., von Gersdorff, H., & Calvo, D. J. (2011). Allosteric modulation of retinal GABA receptors by ascorbic acid. Journal of Neuroscience, 31(26), 9672. doi:10.1523/JNEUROSCI.5157-10.2011
- Rasmussen, H. M., & Johnson, E. J. (2013). Nutrients for the aging eye. Clinical Interventions in Aging, 8, 741–748. https://doi.org/10.2147/CIA.S45399
- Gombart, A. F., Pierre, A., & Maggini, S. (2020). A review of micronutrients and the immune system – working harmony to reduce risk of infection. Nutrients, 12, 236. doi:10.390/nu12010236
Media Attributions
- Illu_thyroid_parathyroid
- Blood Calcium Regulation
- The Functions of Vitamin D
- Peak Bone Mass
Condition of soft, weak, deformed bones in children caused by a deficiency of vitamin D
low bone density in adults
Protein on red blood cells that transports oxygen
Oxygen-carrier protein in muscle
Form of iron that is most easily absorbed, found only in animal foods.
Form of iron not well absorbed that is found in both plant and animal foods
Condition caused by a genetic mutation that leads to abnormal iron metabolism. Iron accumulates in organs such as liver, pancreas, and heart and can cause serious symptoms such as impaired pancreatic and liver function.
Most common nutrient deficiency disease in the world, caused by inadequate iron levels in the body. Common symptoms are fatigue, weakness, pale skin, dizziness, and shortness of breath.
digestive enzymes released from salivary glands that begins the digestion of lipids in infants
The formation of glycogen from excess glucose molecules, occurring in the liver and muscle cells
Nutrients added to a food product that would not naturally occur in that product.
Preventable and reversible condition of inability to see clearly in low light situations. Can be caused by vitamin A deficiency.
Severe drying of the eyes caused by a deficiency of vitamin A
