Chapter 6: Protein
“Life expectancy would grow by leaps and bounds if vegetables smelled as good as bacon.”
-Doug Larson, American Journalist (1926-2017)
Protein makes up approximately 20% of the human body and is present in every single cell. The word protein is a Greek word meaning “of utmost importance.” Proteins are called the workhorses of life as they provide the body with structure and perform a vast array of functions. You can stand, walk, run, skate, swim, and more because of your protein-rich muscles. Protein is necessary for proper immune system function, digestion, hair and nail growth, and is involved in numerous other body functions. In fact, it is estimated that more than 20,000 different proteins exist within the human body. All structures and most secretions like hormones, enzymes, and neurotransmitters in the human body are made from protein. In this chapter you will learn about the components of protein, the important roles that protein serves within the body, how the body uses protein, the risks and consequences associated with too much or too little protein, and where to find healthy sources of it in your diet.
Learning Objectives
- Describe the roles, structure, and functions of proteins.
- Explain the process of denaturation and its consequences.
- Describe how proteins are digested and absorbed by our bodies.
- Describe protein synthesis and the health consequences of protein imbalance.
- Calculate your recommended protein intake using the RDA for protein and the AMDR.
- Explain the ways to determine protein quality.
- Describe the various types of vegetarianism and strategies for vegetarians to consume adequate protein.
- Differentiate between a food allergy and a food intolerance and list top food allergens.
6.1 Defining Protein
Proteins are macromolecules composed of amino acids. Proteins are crucial for the nourishment, renewal, and continuance of life. Proteins contain the elements carbon, hydrogen, and oxygen just as carbohydrates and lipids do, but proteins are the only macronutrients that also contain nitrogen. Amino acids are commonly called protein’s building blocks. There are 20 standard amino acids important for making body proteins. The elements in each amino acid are arranged into a specific conformation around a carbon center. Each amino acid consists of a central carbon atom connected to a side chain (R), a hydrogen ion (H), a nitrogen-containing amino group (NH2), and a carboxylic acid group (COOH)—hence the name “amino acid.”

It’s All in the Side Chain
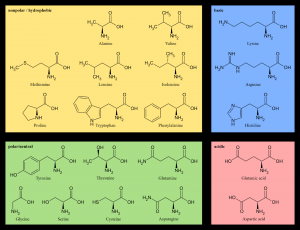
The side chain of an amino acid, often called the “R” group (see Figure 6.1.1), can be as simple as one hydrogen bonded to the carbon center, or as complex as a six-carbon ring bonded to the carbon center. Because the “baseline” of all of the amino acids is the same, what differentiates one amino acid from another is its side chain. Figure 6.1.2 shows the chemical structures of common amino acids.
Essential and Nonessential Amino Acids
Amino acids are further classified based on nutritional aspects. Recall that there are 20 different standard amino acids, and we require all of them to make the thousands of different proteins found throughout the body. Nine of the amino acids are called essential amino acids because we cannot synthesize them either at all or in sufficient amounts to sustain life. These must be obtained from the diet. The other eleven are called nonessential amino acids because the body can synthesize them as long as adequate essential amino acids are consumed. Sometimes during infancy, growth, and in disease states, the body cannot synthesize enough of some of the nonessential amino acids and more of them are required in the diet. These are then called conditionally essential amino acids. The nutritional value of a protein is dependent on which types of amino acids it contains and in what quantities.
Table 6.1.1 Standard Essential and Nonessential Amino Acids (*denotes Conditionally Essential)
| Essential Amino Acids | Nonessential Amino Acids |
| Histidine | Alanine |
| Isoleucine | Arginine |
| Leucine | Asparagine |
| Lysine | Aspartic Acid |
| Methionine | Cysteine* |
| Phenylalanine | Glutamic Acid |
| Threonine | Glutamine* |
| Tryptophan | Glycine* |
| Valine | Proline* |
| Serine | |
| Tyrosine* |
The Many Different Types of Proteins
As previously mentioned, there are over 20,000 different proteins in the human body.1 Different proteins are produced because there are 20 amino acids that are combined in unique sequences. Additionally, proteins come in many different sizes. The hormone insulin, which helps regulate blood glucose, is composed of only 51 amino acids; whereas collagen, a protein that acts like glue between cells, consists of more than 1,000 amino acids. Titin is the largest known protein. It accounts for the elasticity of muscles, and consists of more than 25,000 amino acids!
The abundant variations of proteins are due to the unending number of amino acid sequences that can be formed. To compare how so many different proteins can be designed from only 20 amino acids, think about music. All of the music that exists in the world has been derived from a basic set of seven notes A, B C, D, E, F, G and variations thereof. As a result, there is a vast array of music and songs all composed of specific sequences from these basic musical notes. Similarly, 20 amino acids can be linked together in an extraordinary number of sequences, much more than are possible for the seven musical notes to create songs. As a result, there are enormous variations and potential amino acid sequences that can be created. For example, if an amino acid sequence for a protein is 104 amino acids long the possible combinations of amino acid sequences is equal to 20104, which is 2 followed by 135 zeros!
Building Proteins with Amino Acids (Protein Synthesis)
Proteins are constructed in the body’s cells. This process is called protein synthesis. It occurs in two stages called transcription and translation. Making a protein is like making a cake. The recipe for a protein is held within the DNA of the cell’s nucleus. During transcription, the gene recipe from DNA is copied onto a molecule called messenger RNA (mRNA). The mRNA leaves the nucleus and travels to the ribosome of the cell where translation occurs. The genetic code or recipe is read, amino acids are combined via dehydration synthesis, and the protein is made. The ingredients are the amino acids listed in the recipe with the appropriate amounts indicated, and the construction of the protein occurs in the ribosome of a cell like it occurs in the mixing bowl when you make a cake. The amino acids must be connected in a specific order to make each unique protein.
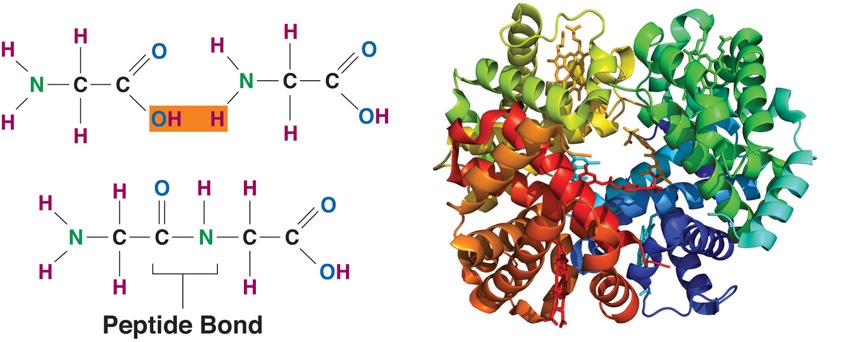
Connecting amino acids with peptide bonds builds proteins, which can also be called polypeptides. Amino acids are sequentially strung along in a chain in a specific sequence that spontaneously folds into the correct protein shape based on the positive, negative, or neutral charges of the amino acid R groups. These sequences contain all the information necessary to fold into a particular shape. A change in the amino acid sequence will cause a change in protein shape, which will prevent the protein from functioning correctly. Each protein in the human body differs in its amino acid sequence and consequently, its shape. The newly synthesized protein is structured to perform a particular function in a cell. A protein made with an incorrectly placed amino acid may not fold the same way, which will change the shape of the protein. This in turn means the protein would not function properly, which can sometimes cause disease.
Food For Thought
There are over 4,000 known diseases caused by incorrectly built proteins. Find out more about how one incorrectly placed amino acid causes the disease sickle cell anemia by watching this animation.
DNA Learning Center – Sickle Cell Anemia
(DNA Learning Center. (n.d.) Sickle Cell Anemia. Cold Spring Harbor Laboratory, www.dnai.org, CC:BY)
Protein Organization
The shape of a protein determines its function. Proteins are similar to carbohydrates and lipids in that they are polymers of simple repeating units; however, proteins are much more structurally complex. In contrast to carbohydrates, which have identical repeating units, proteins are made up of amino acids that are different from one another. A protein’s structure also influences its nutritional quality. Large fibrous protein structures are more difficult to digest than smaller proteins and some, such as keratin, are indigestible. Because digestion of some fibrous proteins is incomplete, not all of the amino acids are absorbed and available for the body to utilize, thereby decreasing their nutritional value.
Cooking and Denaturation
In addition to having many vital functions within the body, proteins perform different roles in our foods by adding certain functional qualities to them. Protein provides food with structure and texture and enables water retention. For example, proteins foam when agitated. Picture whisking egg whites to make angel food cake. The foam bubbles are what give the angel food cake its airy texture. Yogurt is another good example of proteins providing texture. Milk proteins called casein coagulate, increase yogurt’s thickness. Cooked proteins add some color to foods as the amino group binds with carbohydrates and produces a brown pigment. Eggs are 10-15% protein by weight. Most cake recipes use eggs because the egg proteins help bind all the other ingredients together into a uniform cake batter. The proteins aggregate into a network during mixing and baking that gives cake structure.
Protein Denaturation: Unraveling the Fold
When a cake is baked, the proteins are denatured. Denaturation refers to the physical changes that take place in a protein exposed to abnormal conditions in the environment. Heat, acid, high salt concentrations, alcohol, and mechanical agitation can cause proteins to denature. When a protein denatures, its complicated folded structure unravels, and it becomes just a long strand of amino acids again. Weak chemical forces that hold the folds of the protein are broken when a protein is exposed to these conditions. Because a protein’s function is dependent on its shape, denatured proteins are no longer functional. During cooking the applied heat causes proteins to vibrate. This destroys the weak bonds holding proteins in their complex shape (though this does not happen to the stronger peptide bonds that hold the individual amino acids together in a chain). The unraveled protein strands then stick together, forming an aggregate (or network).
Video 6.1.1 Denaturing (Breaking) Protein
Watch this six minute video about what happens when proteins are denatured. As you’re watching, think about how this is happening in your own stomach when proteins are exposed to hydrochloric acid, and how denaturing proteins helps us to digest them.
(Sayres, M.W. (2018). Breaking Protein. Department of Biology, AskABiologist, Arizona State University, CC:BY)
6.2 Protein Digestion and Absorption
How do the proteins from foods, denatured or not, get processed into amino acids that cells can use to make new proteins? When you eat food the body’s digestive system breaks down the protein into the individual amino acids via hydrolysis, turning the large macromolecule called a polypeptide into smaller polypeptides, then into tri- and dipeptides (having only three and two amino acids, respectively), and then into individual amino acids. The individual amino acids are absorbed and used by cells to build other proteins and a few other macromolecules, such as DNA. We discussed the process of food digestion in depth in Chapter 4, but now let’s follow the specific path that proteins take down the gastrointestinal tract and into the circulatory system. Eggs are a good dietary source of protein and will be used as our example to describe the path of proteins in the processes of digestion and absorption. One egg, whether raw, hard-boiled, scrambled, or fried, supplies about six grams of protein.
From the Mouth to the Stomach
Unless you are swallowing it raw, the first step in egg digestion (or any other protein food) involves mastication (chewing). The teeth begin the mechanical breakdown of the large egg pieces into smaller pieces, becoming a bolus that can be swallowed. The salivary glands provide some fluids via saliva to aid swallowing and the passage of the partially mashed egg through the esophagus. Peristalsis and segmentation help propel the egg through the esophagus. The mashed egg pieces enter the stomach through the lower esophageal sphincter. The stomach releases gastric juices containing hydrochloric acid and the proenzyme pepsinogen. The hydrochloric acid of the stomach denatures (or unfolds) the large polypeptide proteins and helps break down the protein aggregates formed during cooking. It also activates the proenzyme pepsinogen and turns it into pepsin, the first protein digesting enzyme. Pepsin splits the large protein chains into smaller and smaller fragments (smaller polypeptides). Egg proteins are large globular molecules and their chemical breakdown requires time and mixing. Powerful stomach contractions churn the partially digested protein into a more uniform mixture. It mixes with other partially digested macromolecules, becoming chyme. Protein digestion in the stomach takes longer than carbohydrate digestion, but less time than fat digestion. A high protein meal remains in the stomach longer, making you feel full (or satiated) longer.
From the Stomach to the Small Intestine
The stomach empties the chyme containing the broken down egg pieces (smaller polypeptides) through the pyloric sphincter and into the small intestine, where the majority of enzymatic protein digestion occurs. As the chyme enters the duodenum, cells in the duodenum release two hormones, secretin and cholecystokinin (CCK). Secretin travels to the pancreas, signaling it to secrete bicarbonate, a basic fluid that neutralizes any gastric acid within the chyme, and inactivates pepsin. Secretin and CCK also signal the release of pancreatic juice containing more protein digesting enzymes called (proteases) into the duodenum. These enzymes further break down the protein fragments. The two major pancreatic proteases, chymotrypsin and trypsin break down the smaller polypeptides into very small chains of amino acids containing only three or two amino acids called tri- and dipeptides. The cells that line the small intestine release additional enzymes called (peptidases) that finally split the tri- and dipeptides into individual amino acids.
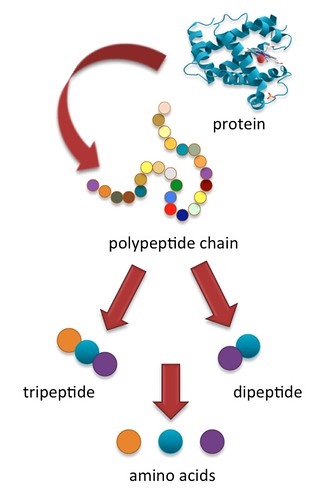
The peristaltic muscle contractions of the small intestine mix and propel the amino acids to the absorption sites. In the jejunum and ileum of the small intestine, the amino acids are transported from the intestinal lumen through the intestinal cells to the blood. This movement of individual amino acids occurs via active transport, requiring special transport proteins and the cellular energy molecule, adenosine triphosphate (ATP).
Once the amino acids are in the blood, they are transported to the liver for further processing. Essential amino acids can be transformed into nonessential amino acids, a process called transamination. Amino acids can also be converted to glucose (gluconeogenesis) or to fat (lipogenesis). To do this, the liver must remove the nitrogen on the amino acid (deamination). The nitrogen is converted to ammonia. To protect the body from this harmful, toxic substance, the liver transforms ammonia to urea which is then transported to the kidney and excreted in the urine. What remains are the carbon, hydrogen, and oxygen atoms which can become glucose and used by the body for energy, or stored as body fat.
Table 6.2.1 Summary of Secretions and their Functions in Protein Digestion
| Organ | Secretion | Function |
| Salivary Glands | Saliva | Moisten and soften food |
| Stomach | Gastrin (hormone) | Turns on hydrochloric acid pumps |
| Hydrochloric acid | 1. Denatures (unravels) protein strands 2. Activates pepsinogen into pepsin (enzyme) |
|
| Pepsin (enzyme) | Begins to split large polypeptides into smaller polypeptides | |
| Small Intestine | Secretin and CCK (hormones) | Signal pancreas to release secretions |
| Pancreas | Proteases: Chymotrypsin and Trypsin (enzymes) | Break down smaller polypeptides into tri- and dipeptides |
| Bicarbonate | Neutralizes the hydrochloric acid in the chyme | |
| Intestinal villi | Peptidases (enzymes) | Break down tri- and dipeptides into individual amino acids |
Amino Acids Are Recycled
All cells in the body continually break down proteins and build new ones, a process referred to as protein turnover. Every day over 250 grams of protein in your body are dismantled and 250 grams of new protein are built. To form these new proteins, amino acids from food and those from protein destruction are placed into an amino acid pool. Though it is not a literal pool, when an amino acid is required to build another protein it can be acquired from the additional amino acids that exist within the body. Amino acids are used not only to build proteins, but also to build other biological molecules containing nitrogen, such as DNA and RNA, and to a lesser extent to produce energy.
It is critical to maintain amino acid levels within this cellular pool by consuming high quality proteins in the diet, or the amino acids needed for building new proteins will be obtained by increasing protein destruction from other tissues within the body, especially muscle. If a needed amino acid is unavailable, either from the diet or from body protein destruction, the construction of the new protein will stop. This might occur if dietary intake of protein is limited, and will be discussed in more detail later in this chapter. This amino acid pool is less than 1% of total body protein content. Thus, the body does not store protein as it does carbohydrates (as glycogen in the muscles and liver) and lipids (as triglycerides in adipose tissue).
6.3 Functions of Protein in the Body
Proteins come in all sizes and shapes. They are the “workhorses” of the body and participate in many bodily functions. Each protein is unique and is specifically structured for its particular function.
Structure and Motion
More than 100 different structural proteins have been discovered in the human body, but the most abundant by far is collagen, which makes up about 6% of total body weight. Collagen makes up 30% of bone tissue and comprises large amounts of tendons, ligaments, cartilage, skin, and muscle. Collagen is a strong, fibrous protein made up of mostly glycine and proline amino acids. Within its structure three protein strands twist around each other like a rope and then these collagen ropes overlap with others. This highly ordered structure is even stronger than steel fibers of the same size.
Collagen makes bones strong, but flexible. Collagen fibers in the skin provide it with structure, and the accompanying elastin protein fibrils make it flexible. Pinch the skin on the back of your hand and then let go; the collagen and elastin proteins in skin allow it to go back to its original shape. Smooth muscle cells that secrete collagen and elastin proteins surround blood vessels, providing the vessels with structure and the ability to stretch back after blood is pumped through them. Another strong, fibrous protein is keratin, which is a primary structural element of skin, hair, and nails.
The closely packed collagen fibrils in tendons and ligaments allow for synchronous mechanical movements of bones and muscle and the ability of these tissues to spring back after a movement is complete. Move your fingers and watch the synchrony of your knuckle movements. In order to move, muscles must contract. The contractile parts of muscles are the proteins actin and myosin. When these proteins are stimulated by a nerve impulse they slide across each other, causing a shortening of the muscle cell. Upon stimulation, multiple muscle cells shorten at the same time, resulting in muscle contraction.
Enzymes
Although proteins are found in the greatest amounts in connective tissues such as bone, their most extraordinary function is as enzymes. Enzymes are proteins that conduct specific chemical reactions. An enzyme’s job is to provide a site for a chemical reaction and to lower the amount of energy and time it takes for that chemical reaction to happen (this is known as “catalysis”). On average, more than 100 chemical reactions occur in cells every single second and most of them require enzymes. The liver alone contains over 1,000 enzyme systems. Enzymes are specific and will use only particular substrates that fit into their active site, similar to the way a lock can be opened only with a specific key. Nearly every chemical reaction requires a specific enzyme. Fortunately, an enzyme can fulfill its role as a catalyst over and over again, although eventually it is destroyed and rebuilt. All bodily functions, including the breakdown of macronutrients in the stomach and small intestine, the transformation of nutrients into molecules a cell can use, and building all macromolecules, including protein itself, involve enzymes.
Hormones
Proteins are responsible for hormone synthesis. Recall that hormones are the chemical messengers produced by the endocrine glands. When an endocrine gland is stimulated, it releases a hormone. The hormone is then transported in the blood to its target cell, where it communicates a message to initiate a specific reaction or cellular process. For instance, after you eat a meal, your blood glucose levels rise. In response to the increased blood glucose, the pancreas releases the hormone insulin. Insulin tells the cells of the body that glucose is available and to take it up from the blood and store it or use it for making energy or building macromolecules. A major function of hormones is to turn enzymes on and off, so some proteins can even regulate the actions of other proteins. While not all hormones are made from proteins, many of them are.
Fluid and Acid-Base Balance
Proper protein intake enables the basic biological processes of the body to maintain the status quo in a changing environment. Fluid balance refers to maintaining the distribution of water in the body. If too much water in the blood suddenly moves into a tissue, the results are swelling and, potentially, cell death. Water always flows passively from an area of high concentration to one of lower concentration. As a result, water moves toward areas that have higher concentrations of other solutes, such as proteins and glucose. To keep the water evenly distributed between blood and cells, proteins continuously circulate at high concentrations in the blood. The most abundant protein in blood is the butterfly-shaped protein known as albumin. Albumin’s presence in the blood makes the protein concentration in the blood similar to that in cells. Therefore, fluid exchange between the blood and cells is minimized to preserve the status quo.
Protein is also essential in maintaining proper pH balance (the measure of how acidic or basic a substance is) in the blood. Blood pH is maintained between 7.35 and 7.45, which is slightly basic. Even a slight change in blood pH can affect body functions. Recall that acidic conditions can cause protein denaturation, which stops proteins from functioning. The body has several systems that hold the blood pH within the normal range to prevent this from happening. One of these is the circulating albumin. Albumin is slightly acidic, and because it is negatively charged it balances the many positively charged molecules, such as hydrogen protons (H+), calcium (Ca2+), potassium (K+), and magnesium (Mg2+) which are also circulating in the blood. Albumin acts as a buffer against abrupt changes in the concentrations of these molecules, thereby balancing blood pH and maintaining homeostasis. The protein hemoglobin also participates in acid-base balance by binding hydrogen protons.
Transport
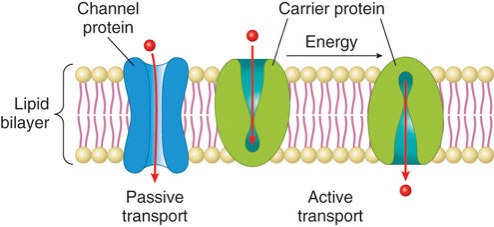
Albumin and hemoglobin also play a role in molecular transport. Albumin chemically binds to hormones, fatty acids, some vitamins, minerals, and drugs, and transports them throughout the circulatory system. Each red blood cell contains millions of hemoglobin molecules that bind oxygen in the lungs and transports it to all the tissues in the body. A cell’s plasma membrane is usually not permeable to large polar molecules, so to get the required nutrients and molecules into the cell many transport proteins exist in the cell membrane. Some of these proteins are channels that allow particular molecules to move in and out of cells passively through a process called facilitated diffusion. Others act as one way taxis and require energy to function, a process called active transport.
Protection and Immunity
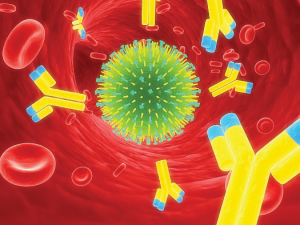
Earlier we discussed that the strong collagen fibers in skin provide it with structure and support. The skin’s dense network of collagen fibers also serves as a barricade against harmful substances. The immune systems’ attack and destroy functions are dependent on enzymes and antibodies, which are also proteins. An enzyme called lysozyme is secreted in the saliva and attacks the walls of bacteria in the mouth causing them to rupture. Certain proteins circulating in the blood can be directed to build a molecular knife that stabs the cellular membranes of foreign invaders. The antibodies secreted by the white blood cells survey the entire circulatory system looking for harmful bacteria and viruses to surround and destroy. Antibodies also trigger other factors in the immune system to seek and destroy unwanted intruders.
Food For Thought
Given protein’s critical roles in the immune system, what do you think might occur more frequently in a person whose diet is protein deficient?
Video 6.3.1 Specific Immunity: What are Antibodies?
Watch this short video to observe how antibodies protect against foreign intruders.
(FuseSchool-Global Education. (2018) What are Antibodies? CC:BY:NC)
Wound Healing and Tissue Regeneration
Proteins are involved in all aspects of wound healing, a process that takes place in three phases: inflammatory, proliferation, and remodeling. For example, if you were sewing and pricked your finger with a needle, your flesh would turn red and become inflamed. Within a few seconds bleeding would stop. The healing process begins with proteins such as bradykinin, which dilate blood vessels at the site of injury. An additional protein called fibrin helps to secure platelets that form a clot to stop the bleeding. Next, in the proliferative phase, cells move in and mend the injured tissue by installing newly made collagen fibers. The collagen fibers help pull the wound edges together. In the remodeling phase, more collagen is deposited, forming a scar. Scar tissue is only about 80% as functional as normal uninjured tissue. If a diet is insufficient in protein, the process of wound healing is markedly slowed.
While wound healing takes place only after an injury is sustained, a different process called tissue regeneration is ongoing in the body. The main difference between wound healing and tissue regeneration is in the product formed. The process of regeneration creates an exact structural and functional copy of the lost tissue. Thus, old, dying tissue is not replaced with scar tissue but with brand new, fully functional tissue. Some cells (such as skin, hair, nails, and intestinal cells) have a very high rate of regeneration, while others, such as heart muscle cells and nerve cells do not regenerate at any appreciable levels. Tissue regeneration is the creation of new cells (cell division), which requires many different proteins including enzymes that synthesize RNA and proteins, transport proteins, hormones, and collagen. In a hair follicle, cells divide and a hair grows in length. Hair growth averages 1 centimeter per month and fingernails about 1 centimeter every 100 days. The cells lining the intestine regenerate every 3-5 days. Diets deficient in protein impair tissue regeneration causing many health problems including impairment of nutrient digestion and absorption and, most visibly, a reduction in hair and nail growth.
Energy Production
Some of the amino acids in proteins can be disassembled and used to make energy. The liver is able to break down amino acids by removing the nitrogen. The nitrogen becomes ammonia and then urea which is excreted in urine. What remains is the carbon skeleton which can then be fed into the catabolic Kreb’s cycle to generate energy in the form of ATP. Only about 10% of dietary proteins are catabolized each day to make cellular energy directly. If a person’s diet does not contain enough carbohydrates and fats, their body will use more amino acids to make energy, which compromises the synthesis of new proteins and breaks down muscle proteins. Not only can some amino acids be used for energy directly, recall from Chapter 5 that they can also be used to synthesize glucose through gluconeogenesis.
If a person’s diet contains more protein than the body needs, the extra amino acids will be broken down, the nitrogen removed, and the carbon skeletons transformed into fat and stored. The nitrogen becomes a toxic product called ammonia. In order to reduce ammonia, the liver converts it to less toxic urea, which is released into the blood, filtered by the kidneys, and excreted in urine.
6.4 Protein, Diet, and Personal Choices
We have discussed what proteins are, how they are made, how they are digested and absorbed, and the many functions of proteins in the body. This section will provide you with information on how to determine the recommended amount of protein for you, and your many choices in designing an optimal diet with high quality protein sources.
How Much Protein Does a Person Need?
The appropriate amount of protein in a person’s diet is that which maintains a balance between what is taken in and what is used (homeostasis). The RDA for protein was determined by assessing nitrogen balance (nitrogen input through foods and beverages vs nitrogen output). Remember that when amino acids are broken down, nitrogen is removed. Most is lost as urea in the urine, but urea is also excreted in the feces. Proteins are also lost in sweat and as hair and nails grow. The RDA, therefore, is the amount of protein a person should consume in their diet to balance the amount of protein used up and lost from the body. For healthy adults, this amount of protein is determined to be 0.8 g of protein per kg of body weight per day. You can calculate your exact recommended protein intake per day based on your weight by using the following equation:
(Weight in lbs. ÷ 2.2 lb/kg) × 0.8 g/kg
For example, if you weigh 175 lb, your recommended protein intake would be:
175 lb/2.2 = 79.5 kg
79.5 kg x 0.8 g/kg = 63.6 or ~64 g protein per day
The National Academy of Medicine (NAM) used data from multiple studies that determined nitrogen balance in people of different age groups to calculate the RDA for protein. A person is said to be in nitrogen balance when the nitrogen input equals the amount of nitrogen used and excreted. A person is in negative nitrogen balance when the amount of excreted nitrogen is greater than that consumed, meaning that the body is breaking down more protein to meet its demands. This state of imbalance can occur in people who have certain diseases, such as cancer or muscular dystrophy. Someone who has a low protein diet may also be in negative nitrogen balance as they are taking in less protein than what they actually need. Positive nitrogen balance occurs when a person excretes less nitrogen than what is taken in by the diet, such as during child growth or pregnancy. At these times the body requires more protein to build new tissues, so more of what gets consumed gets used up and less nitrogen is excreted. A person healing from a severe wound may also be in positive nitrogen balance because protein is being used up to repair tissues.
Table 6.4.1 Nitrogen Balance
| Nitrogen Balance | Nitrogen in = Nitrogen out | Homeostasis |
| Negative Nitrogen Balance | Nitrogen in < Nitrogen out | Significant illness (cancer, HIV), trauma, burns |
| Positive Nitrogen Balance | Nitrogen in > Nitrogen out | Periods of growth (pregnancy, childhood) |
Another way to calculate the amount of protein that should be consumed in the diet is to use the AMDR for protein which is 10-35% of total kcal. Let’s say that you are consuming an average of 1800 kcal/day. Using the AMDR, you would calculate the kcal from protein, and then convert it to grams of protein by dividing by 4 kcal/g.
1800 x 0.10 = 180 kcal protein/4 kcal per gram = 45 g protein
1800 x 0.35 = 630 kcal protein/4 kcals per gram = 157 g protein
Depending on the other macronutrients you are consuming, you would need 45-157 g of protein per day.
You could also use the recommendations from ChooseMyPlate which provides guidance in ounces (oz) or ounce-equivalents (oz-equiv) which are listed in Table 6.4.2.
Table 6.4.2 ChooseMyPlate Daily Protein Foods Table Daily Recommendation* in ounce-equivalents (oz-equiv)**
| Women | 19-30 years 31-50 years 51+ years |
5 ½ oz-equiv 5 oz-equiv 5 oz-equiv |
| Men | 19-30 years 31-50 years 51+ years |
6 ½ oz-equiv 6 oz-equiv 5 ½ oz-equiv |
*These amounts are appropriate for individuals who get less than 30 minutes per day of moderate physical activity, beyond normal daily activities. Those who are more physically active may be able to consume more while staying within calorie needs.
**1 oz of meat, poultry or fish, ¼ c cooked beans, 1 egg, 1 tbs of peanut butter, or ½ oz of nuts or seeds can be considered as 1 oz-equiv from the Protein Foods Group.2
Dietary Sources of Protein
The protein food group within ChooseMyPlate consists of foods made from meat, seafood, poultry, eggs, soy, beans, peas, nuts, and seeds, but protein can be found in every food group on ChooseMyPlate in varying amounts. Protein sources differ in their additional components, so it is necessary to pay attention to the whole nutrient “package.” Protein-rich animal-based foods commonly have high amounts of B vitamins, vitamin E, iron, magnesium, and zinc, but some may also contain an unhealthy amount of saturated fat and cholesterol. Seafood often contains healthy fats, and plant sources of protein contain a high amount of fiber. When choosing your dietary sources of protein, take note of the other nutrients in order to make good selections that will benefit your health and fit into an overall healthy eating pattern.
Choosing Protein Sources
A hamburger patty made from 80% lean meat contains 22 g of protein, 5.7 g of saturated fat, 77 mg of cholesterol, and 230 kcal.
A hamburger patty made from 95% lean meat also contains 22 g of protein, but has 2.3 g of saturated fat, 60 mg of cholesterol, and 139 kcal.
A cup of boiled soybeans contains 29 g of protein, 2.2 g of saturated fat, no cholesterol, and 298 kcal.
Which of these would you choose? Remember from the discussion in Chapter 2 that food choices are complicated. Maybe you’ll choose soybeans because you are a vegetarian or have high blood cholesterol levels, even though it has the highest number of calories. Maybe you’ll choose the hamburger made with 95% lean beef because you want fewer calories and less saturated fat. Each person must choose what they ingest based on their personal circumstances, but if you have more information you can make a more informed choice. Table 6.4.3 provides more nutrition information about popular sources of protein in the American diet.
Table 6.4.3 Sources of Dietary Protein with Selected Nutrient and Calorie content
| Food | Protein Content (g) | Saturated Fat (g) | Cholesterol (mg) | Calories |
| Pork ribs (1 piece) | 56.4 | 22.2 | 222 | 790 |
| Chicken breast (1 c, roasted) | 43.4 | 1.4 | 119 | 231 |
| Chicken leg (1 leg) | 29.6 | 4.2 | 105 | 264 |
| Top sirloin (3 oz) | 25.8 | 2.0 | 76 | 158 |
| Pork loin (3 oz) | 24.3 | 3.0 | 69 | 178 |
| Beef chuck (3 oz, lean, trimmed) | 22.2 | 1.8 | 51 | 135 |
| Tilapia (3 oz) | 22.2 | 0.8 | 48 | 109 |
| Tuna (3 oz, canned) | 21.7 | 0.2 | 26 | 99 |
| Shrimp (breaded, fried, 6-8 pieces) | 18.9 | 5.4 | 200 | 454 |
| Salmon (3 oz) | 18.8 | 2.1 | 54 | 175 |
| Lentils (1 c, boiled) | 17.9 | 0.1 | 0 | 226 |
| Shrimp (3 oz) | 17.8 | 0.2 | 166 | 84 |
| Chicken thigh (1 thigh, roasted) | 13.5 | 1.6 | 49 | 109 |
| Kidney beans (1 c, canned) | 13.5 | 0.2 | 0 | 215 |
| Sunflower Seeds (1 c) | 9.6 | 2.0 | 0 | 269 |
The US Department of Agriculture (USDA) provides some tips for choosing your dietary protein sources. Their motto is “Go Lean with Protein.” The overall suggestion is to eat a variety of protein-rich foods to benefit health. The USDA recommends that you:
- Choose lean meats, such as round steaks, top sirloin, extra lean ground beef, pork loin, and skinless chicken.
- Eat 8 oz of cooked seafood every week (typically as two 4 oz servings) to ensure you are getting the healthy omega-3 fatty acids that have been linked to a lower risk for heart disease.
- Eat beans, peas, or soy products as a main dish. Such menu choices could include chili with kidney and pinto beans, hummus on pita bread or with raw vegetables, and black bean enchiladas.
- Enjoy nuts in a variety of ways. You can put them on a salad, in a stir-fry, or use them as a topping for steamed vegetables in place of meat or cheese.
If you do not eat meat, the USDA has much more information on how to get all the protein you need from a plant-based diet. When choosing the best protein-rich foods to eat, pay attention to the whole nutrient package and remember to select from a variety of protein sources to get all the other essential micronutrients.
Interactive 6.4.1
The USDA manages the website choosemyplate.gov. The site provides a lot of tips and information about protein-rich foods. You can also download the MyPlate Mobile App which can help you set and reach dietary goals.
Protein Quality
While protein is contained in a wide variety of foods, it differs in quality. High-quality protein contains all the essential amino acids in the proportions needed by the human body. The amino acid profile of different foods is therefore one component of protein quality. Foods that contain only some of the essential amino acids or all of the essential amino acids but in small amounts are called incomplete protein sources, while those that contain all nine essential amino acids in relatively high amounts are called complete protein sources, or high-quality protein sources. Foods that are complete protein sources include animal foods such as milk, cheese, eggs, fish, poultry, and meat, and a few plant foods, such as soy, quinoa, and pistachios. The only animal-based protein that is incomplete is gelatin, which consists of the protein, collagen.

Most plant-based foods are deficient in at least one essential amino acid and therefore are incomplete protein sources. For example, grains are usually deficient in the amino acid lysine, and legumes do not contain enough methionine or tryptophan. Because grains and legumes are not deficient in the same amino acids they can complement each other in a diet. Incomplete protein foods are called complementary foods because when consumed in tandem they contain all nine essential amino acids at adequate levels. Generally, consuming a legume or bean with a grain or nut will provide the nine essential amino acids. Complementary protein sources do not have to be consumed at the same time—as long as they are consumed within the same day, you will meet your protein needs. One example of two complementary plant foods is rice and beans. Another is peanut butter on 100% whole wheat bread.
The second component of protein quality is digestibility, as not all protein sources are equally digested. In general, animal-based proteins are completely broken down during the process of digestion, whereas plant-based proteins are not. This is because some proteins are contained in the plant’s fibrous cell walls and, as discussed previously, these fibers pass through the digestive tract unabsorbed by the body.
If consumption of all nine essential amino acids is inadequate, protein synthesis will be compromised. The manufacturing of some body proteins may be limited. The amino acid in the shortest supply relative to its requirement is called the limiting amino acid.
Measures of Protein Quality
How do you know the quality of the protein in the foods you consume? The protein quality of most foods has been determined by one of the methods below. These are not terms you would find on food labels, but they are used in food science and research.
Biological Value (BV): (grams of nitrogen retained/grams of nitrogen absorbed) x 100
Efficiency Ratio (PER): (grams of weight gained/grams of protein consumed); This method is commonly performed in growing rats.
Chemical or Amino Acid Score (AAS): [Test food limiting essential amino acid (mg/g protein)/needs of same essential amino acid (mg/g protein)]
Protein Digestibility Corrected Amino Acid Score (PDCAAS): (Amino Acid Score x Digestibility); This is the most widely used method.
Digestible Indispensable Amino Acid Score (DIAAS): DIAAS % = 100 x (mg of digestible dietary indispensable amino acid in 1 g of the dietary protein/mg of the same dietary indispensable amino acid in 1g of the reference protein); Ideal digestibility should be utilized to determine the digestibility in DIAAS (ideally in humans, but if not possible in growing pigs or rats). Currently used by Food and Agriculture Organization of the World Health Organization (WHO).
Protein Needs: Special Considerations
Some groups may need to examine how to meet their protein needs more closely than others. We will take a closer look at the special protein considerations for vegetarians and the elderly.
Vegetarians and Vegans
Plant-based diets are increasing in popularity. There are many good reasons to consider adopting a plant-based diet including a reduction in risk for many chronic diseases. Well planned vegetarian diets can easily meet your nutritional needs. However, just like any eating pattern, the food choices you make should follow the dietary guidelines. A vegetarian diet based on processed foods high in sodium, saturated fats, sugar, and kcal would not be beneficial.
While there are different forms of vegetarianism, the common theme in all is that they do not eat meat. The three most common forms are:
- Vegan: Includes only plant foods. No animal proteins or animal by-products such as eggs, milk, or honey
- Lacto-vegetarian: Includes plant foods plus some or all dairy products. This is the most common form of vegetarianism in the world
- Lacto-ovo vegetarian: Includes plant foods, dairy products, and eggs
Some people follow a semi- or partial vegetarian (sometimes called a flexitarian) diet that includes plant foods and may include poultry or fish, dairy products, and eggs but does not include red meat. For example, a pescatarian typically consumes only plant foods and seafood. While such diets focus on consuming mostly plant foods and minimizing consumption of animal products these types of consumers are not officially recognized as vegetarians.
Interactive 6.4.2
Nutrition Data is a useful resource for determining protein quality and identifying complementary proteins. To use the site, go to nutrition data.self.com, type in the name of the food you would like to know about in the search bar and hit ‘Enter’. When you have selected your food from the list of possibilities, you will be given information about this food. Included in this information is the Protein Quality section. This will give you an amino acid score and a figure that illustrates which amino acid(s) is limiting. If your food is an incomplete protein, you can click “Find foods with a complementary profile.” This will take you to a list of dietary choices that will provide complementary proteins for your food.
Any vegetarian eating pattern can provide sufficient nutrients including protein as long as it is adequate and varied. Vegetarians (with the exception of vegans) consume complete protein sources such as dairy products or eggs in addition to plants, so can easily accumulate the required amounts of all of the essential amino acids. Vegans can also attain their recommended protein intakes if they give a little more attention to high-quality plant-based protein sources. Following a vegetarian diet and getting the recommended protein intake is also made a little more difficult because the digestibility of plant-based protein sources is lower than the digestibility of animal-based protein as discussed previously. That is why it is so important to vary your intake and choose whole, healthful plant foods every day. According to the Mayo Clinic, “To get the most out of a vegetarian diet, choose a variety of healthy plant-based foods, such as whole fruits and vegetables, legumes and nuts, and whole grains. At the same time, cut back on less healthy choices, such as sugar-sweetened beverages, fruit juices, and refined grains. If you need help, a registered dietitian can assist you in creating a vegetarian plan that’s right for you.”3 If you are interested in adopting a more plant-based diet, experts recommend starting slowly. Below are some suggestions for getting started. In addition, as you’re reducing your intake of animal foods, increase your intake of fruits, vegetables, and legumes.
Suggestions for moving to a plant-based dietary pattern:
- Start with one meal. Modify a favorite recipe like chili by removing the beef and adding in additional beans, or try meatless stir fry or spaghetti with marinara sauce and vegetables
- Choose one day of the week to be meatless (Meatless Monday perhaps?)
- Try ethnic restaurants—they often have more vegetarian options to try
- Pack plant-based snacks when you go to work or school (raw veggies like carrots, celery, peppers, tomatoes; fruits like apple slices, banana, orange slices)
- Check out the internet or vegetarian cookbooks for new recipes to try
Getting All the Nutrients You Need—The Plant-Based Way
Below are five ways to assure you are getting all the nutrients you need while working toward a more plant-based diet:
- Get your protein from foods such as soybeans, tofu, tempeh, lentils, and beans, beans, and more beans. Many of these foods are high in zinc too.
- Eat foods fortified with vitamins B12 and D, and the mineral calcium. Some examples are soy milk and fortified cereals.
- Get enough iron in your diet by eating kidney beans, lentils, whole grain cereals, and leafy green vegetables.
- To increase iron absorption, eat foods with vitamin C at the same time.
- Don’t forget that carbohydrates and fats are required in your diet too. Eat whole grain breads, cereals, and pastas. For fats, eat an avocado, add some olive oil to a salad or stir-fry, or spread some nut butter on a bran muffin.
6.5 Diseases Involving Proteins
As you may recall, moderation refers to having the proper amount of a nutrient—neither too little nor too much. A healthy diet incorporates all nutrients in moderation. Low protein intake has several health consequences, and a severe lack of protein in the diet eventually causes death. Although severe protein deficiency is a rare occurrence in children and adults in the US, it is estimated that more than half of the elderly in nursing homes are protein deficient.
The AMDR for protein for adults is 10-35% of kcal, which is a fairly wide range. The percent of protein in the diet that is associated with malnutrition and its health consequences is less than 10%, but this is often accompanied by deficiencies in calories and micronutrients. On the other hand, diets rich in animal-derived protein (>30% of caloric intake) are associated with increased early mortality, cardiovascular disease, colon cancer, and osteoporosis.
Health Consequences of Protein Deficiency
Although severe protein deficiency is rare in the developed world, it is a leading cause of death in children in many poor, underdeveloped countries. There are two main syndromes associated with protein deficiencies: kwashiorkor and marasmus. Kwashiorkor develops when calorie intake is adequate, but protein intake is not. It is primarily a syndrome in children, affecting millions worldwide. When it was first described in 1935, more than 90% of children with kwashiorkor died. Although the associated mortality is slightly lower now, most children still die after the initiation of treatment. The name kwashiorkor comes from a language in Ghana and means, “rejected one.” The syndrome was named because it occurred most commonly in children who had recently been

weaned from the breast, usually because another child had just been born. Subsequently the child was fed watery porridge made from low protein grains, which accounts for the low protein intake. Kwashiorkor is characterized by swelling (edema) of the feet and abdomen, poor skin health, diminished growth, low muscle mass, and liver malfunction. Recall that one of protein’s functional roles in the body is fluid balance. Diets extremely low in protein do not provide enough amino acids for the synthesis of albumin. One of the functions of albumin is to hold water in the blood vessels, so having lower concentrations of blood albumin results in water moving out of the blood vessels and into tissues, causing swelling. The primary symptoms of kwashiorkor include not only swelling, but also diarrhea, fatigue, peeling skin, and irritability. Severe protein deficiency in addition to other micronutrient deficiencies, such as vitamin C, folate (vitamin B9), iodine, and iron all contribute to the many health manifestations of this syndrome.
Children and adults with marasmus neither have enough protein in their diets nor do they take in enough calories. Marasmus affects mostly children below the age of one in poor countries. Body weights of children with marasmus may be up to 80% less than that of a healthy child of the same age. It also occurs in seniors, especially those with low incomes or limited access to calories. Marasmus is a Greek word, meaning “starvation.” It is characterized by an extreme emaciated appearance, poor skin health, and stunted growth. Other symptoms include acute fatigue, hunger, and diarrhea.
Kwashiorkor and marasmus often coexist as a combined syndrome termed marasmic kwashiorkor or protein-energy malnutrition (PEM). Children with the combined syndrome have variable amounts of edema and the characterizations and symptoms of marasmus. Although organ system function is compromised by undernutrition, the ultimate cause of death is usually infection. Undernutrition is intricately linked with suppression of the immune system at multiple levels, so undernourished children commonly die from severe diarrhea and/or pneumonia resulting from bacterial or viral infection. The United Nations Children’s Fund (UNICEF), the most prominent agency with the mission of changing the world to improve children’s lives, reports that in 2017, 1 in 9 people (821 million people) in the world suffer from hunger, 22% of children (151 million) suffer from stunting (too short for their age), and 7.5% (50 million) suffer from wasting (low weight for height).4

Treating malnutrition in developing nations is challenging. However, scientists have developed ready to use therapeutic foods (RUTF) which can help treat severe, acute malnutrition in children under five years old. These RUTFs do not require refrigeration and can stay fresh for up to two years. No mixing is required, each packet comes ready to use, and so they can be given to children even in locations where there is not safe drinking water, access to cooking facilities and/or where fuel is limited. RUTFs cost about 30 cents per packet and provide life-saving nutrition for severely malnourished children.5 Each packet contains about 500 kcals, 13 g protein, 30 g lipid, 45 g carbohydrate, and almost all of the micronutrients required for optimal daily intake.6 More about malnutrition will be discussed in Chapter 11, Food Insecurity.
The Elderly
As we age, muscle mass gradually declines. This is a process referred to as sarcopenia. A person is sarcopenic when their amount of muscle tissue is significantly lower than the average value for a healthy person of the same age. A significantly lower muscle mass is associated with weakness, movement disorders, and a generally poor quality of life. It is estimated that about half the US population of men and women above the age of 80 are sarcopenic. Although the RDA for protein for elderly persons is the same as that for the rest of the adult population, health experts that study protein and aging recommend protein intakes of 1.2-2.0 g per kg for elderly populations to prevent the significant loss of muscle mass specifically in older adults. Ideally this protein would be high-quality with intake spread throughout the day.7 In addition to adequate kcal and protein, physical activity is essential for delaying the onset of sarcopenia and maintaining functionality in the elderly.8
Many elderly have difficulty consuming high quality protein sources such as meats, poultry, or fish. Fixed incomes may not be adequate to purchase these forms of protein, and sometimes problems with teeth or swallowing may minimize the ability to ingest them. Medications may reduce appetite or inhibit digestion or absorption of nutrients. Sources like eggs and dairy can be beneficial as can nuts, seeds, and legumes. However, plant sources require increased intake to meet requirements.
Health Consequences of Too Much Protein in the Diet
An explicit definition of a high-protein diet has not yet been developed by the Food and Nutrition Board of the NAM, but typically diets high in protein are considered as those that derive more than 30% of calories from protein. Many people follow high-protein diets because marketers tout protein’s ability to stimulate weight loss. It is true that consuming a high-protein diet increases weight loss in some people. However, the number of individuals that remain on this type of diet is low and many people who try the diet and stop, regain the weight they had lost. Additionally, there is a scientific hypothesis that there may be health consequences of remaining on a high-protein diet long term, but clinical trials are ongoing to examine this hypothesis further.
As the high-protein diet trend arose so did the intensely debated issue of whether there are any health consequences of eating too much protein. Observational studies conducted in the general population suggest diets high in animal protein, specifically those in which the primary protein source is red meat, are linked to a higher risk for kidney stones, kidney disease, liver malfunction, colorectal cancer, and osteoporosis. However, diets that include lots of red meat are also high in saturated fat and cholesterol, and sometimes linked to unhealthy lifestyles, so it is difficult to conclude that the increased protein content specifically is the culprit.
- Kidney disease. High-protein diets appear to only increase the progression of kidney disease and liver malfunction in people who already have kidney or liver malfunction, and not to cause these problems. However, the prevalence of kidney disorders is relatively high and underdiagnosed.
- Colon cancer and cardiovascular disease. An assessment of more than ten studies performed around the world published in the June 2011 issue of PLoS purports that a high intake of red meat and processed meat is associated with a significant increase in colon cancer risk.9 Another prospective study looked at the meat intakes of half a million people worldwide and followed them for ten years. People with the highest intake of red (beef, pork, lamb, and goat) and processed meats (hot dogs, ham, bacon, sausage, and some deli meats) had a slightly higher risk of dying of cardiovascular disease and cancer than those that consumed the least amount of red meat.10 The exact mechanism of how proteins, specifically those in red and processed meats, may cause disease is not yet known and requires further study.
- Bone Loss. High-protein diets may accelerate bone tissue loss because under some conditions the acids in protein block absorption of calcium in the gut and once in the blood amino acids promote calcium loss from bone; however these effects have not been consistently observed in scientific studies.
Another issue related to high-protein diets is source. Americans consume a significant amount of meat from livestock sources, about 260 lb per person in 2020.11 Common sources are poultry, pork, beef, dairy, eggs, and to a lesser extent, sheep and goat.11 To make this much animal protein affordable, farmers use concentrated feeding operations, feeding corn and soy to the animals to induce rapid growth and development instead of using these crops to feed people. This shift produces a significantly higher carbon footprint and requires greater use of freshwater per pound for animal-based protein than for plant-based protein. Scientists use a ratio to consider animal versus plant sources of protein, and for Americans that ratio is 85:15. A small reduction in total protein intake with a change to 60:40 animal to plant ratio would reduce environmental impacts and may improve health.12
While the scientific community continues its debate about the particulars regarding the health and environmental consequences of too much protein in the diet, protein like all nutrients, must be eaten in proper amounts. Moderation and variety are key strategies to achieving a healthy diet and need to be considered when optimizing protein intake.
Food for Thought
Are you following a high-protein diet (protein >30% of AMDR)? What are some diet changes you can make that are better, more sustainable, and easier to follow for the rest of your life than a high-protein diet?
Food Allergies
Paying attention to the way individuals react to various foods is essential in determining what foods may specifically affect a person adversely. Food allergies are one of the many ways in which different body make-ups affect nutritional concerns. Food allergies affect approximately 5% of children and 4% of adults in the US.13

When someone has a food allergy, the immune system mistakenly attacks a certain kind of food (usually the protein component of a food), such as peanuts, as if it were a threat and immunoglobulin E (IgE) antibodies are produced. When activated by the presence of the allergen (e.g., peanuts) in the system, IgE antibodies release chemicals that cause the symptoms of an allergic reaction. Doctors often test for food allergies by using skin-prick tests or blood tests to look for the presence of IgE antibodies. However, these types of tests can sometimes yield a false positive result. By far, the most valuable test for determining a food allergy is the double-blind placebo-controlled food challenge, which involves administering tiny amounts of the suspected allergy-inducing food(s) orally under observation of a physician in a clinical setting and denoting the signs and symptoms of an allergic response over 3-4 hours. This is the gold standard of allergy testing but it is time consuming and comes with risk of severe allergic reactions.
Food allergy symptoms usually develop within a few minutes to two hours after a person has eaten a food to which they are allergic. These symptoms can range from the annoying to the potentially fatal and include:
- A tingling mouth
- Swelling tongue and/or throat
- Difficulty breathing
- Hives
- Vomiting and/or abdominal cramps
- Drop in blood pressure
- Loss of consciousness
- Death
There are no clear treatments for food allergies. Epinephrine is sometimes required to control severe reactions, and individuals with known and dangerous allergies may get prescriptions for self-injectable devices. The only certain way to avoid allergic reactions to food is to avoid the foods that cause them. Beyond avoidance, this can mean reading food labels carefully, or even calling manufacturers for product information.
The prevalence of food allergies is a complex and growing problem. In response to this situation, the National Institute of Allergy and Infectious Diseases (NIAID) collaborated with 34 professional organizations, federal agencies, and patient advocacy groups to develop a comprehensive guide to diagnosing and managing food allergies and treating acute food allergy reactions. The guide defines various food allergies, allergens, and reactions, provides comprehensive information on the prevalence of different food allergies, tracks the history of food allergies, and reviews medical management techniques for people with food allergies.
A problem often confused with food allergy is food intolerance, which is also an abnormal response to a food product, but differs from an allergy. Common examples are an intolerance to lactose, a sugar found in many milk products (see Chapter 5), and gluten intolerance, or celiac disease, which occurs when the immune system responds abnormally to gluten, a protein component of barley, wheat, and rye (see Chapter 4). However, unlike food allergies, these disorders do not involve IgE antibodies, and symptoms generally only involve the gastrointestinal system (gas, bloating, cramps, diarrhea).14
Food Intolerance Testing
There are several at-home food intolerance or sensitivity tests being advertised these days. These tests measure the immunoglobin G (IgG) antibody response to several different foods. They claim that by removing foods with high IgG levels from your diet you will see improved gastrointestinal health. Some claim to help reduce “brain fog,” headaches, and even the symptoms associated with rheumatoid arthritis and epilepsy.15
However unlike IgE responses (used in food allergy testing), IgG is a memory antibody that does not predict food allergy or intolerance. In fact, the presence of IgG is likely a normal response of the immune system to exposure to food. Higher levels of IgG for foods may simply be associated with increased tolerance to those foods.15
Food sensitivity tests have not been scientifically proven to accomplish what they claim to do. The few studies that have been done include small sample sizes, lack a placebo group, and rely on participant self-report of adherence and outcomes.16
Key Takeaways
- The 20 standard amino acids that combine to form body proteins differ chemically in the molecular composition of their side chains.
- Proteins are polymers of amino acid monomers held together by peptide bonds. In the body, proteins are the building blocks of all structures. In food, protein adds texture and structure.
- Amino acids are categorized based upon their nutritional aspects. Some are nonessential in the diet because the body can synthesize them, while others are essential in the diet because the body cannot make them.
- The shape of a protein determines its function. If the shape changes (denaturation), the protein cannot function. The many shapes and sizes of proteins allow them to perform a vast array of functions, including: acting as enzymes and hormones, and providing for fluid and acid-base balance, transport, protection, wound healing and tissue regeneration, and energy production.
- Mechanical digestion of protein begins in the mouth and continues in the stomach and small intestine. Chemical digestion of protein begins in the stomach with hydrochloric acid denaturing protein and pepsin splitting large polypeptides into smaller polypeptides, and ends in the small intestine when individual amino acids are absorbed.
- The RDA set for protein for adults is 0.8 g/kg of body weight and represents the amount of protein in the diet required to balance the protein that is used up by the body and that is excreted. Vegans, athletes, and the elderly may require more protein than others.
- The protein foods group consists of foods made from meat, seafood, poultry, eggs, soy, beans, peas, and seeds. By determining a food’s amino acid content and the amount of protein that is actually digested and absorbed we can determine that food’s protein quality (complete are high-quality, incomplete are lower quality).
- Protein deficiency can cause kwashiorkor or marasmus in children. Symptoms include swelling, fatigue, skin problems, irritability, muscle wasting, and eventual death from infection.
- The long-term health consequences of high-protein diets have not been adequately studied, although studies suggest a reduction in animal based protein could be beneficial for human health and the environment.
- A food allergy occurs when the immune system attacks a component in a food (usually a protein) and symptoms can occur throughout the body. A food intolerance is a reaction to a component in a food and symptoms are primarily gastrointestinal.
Portions of this chapter were taken from OER Sources listed below:
Jellum, L., Hitzeman, J., Krauss, M., Henderson, S., Harnden, T., Elsberry, C., Ford, G. (2018). Principles of Nutrition Textbook, Second Edition. Nursing and Health Sciences Open Textbooks. 5. https://oer.galileo.usg.edu/health-textbooks/5
Zimmerman, M., & Snow, B. (2012). An Introduction to Nutrition, v. 1.0. https://2012books.lardbucket.org/books/an-introduction-to-nutrition/
Additional References:
- Ponomarenko, E. A, Poverennaya, E. V., Ilgisonis, E. V., Pyatnitskiy, M. A., Kopylov, A. T., Zgoda, V .G., Lisitsa, A. V., & Archakov, A. I. (2016). The size of the human proteome: The width and depth. International Journal of Analytical Chemistry. https://dx.doi.org/10.1155%2F2016%2F7436849
- United States Food and Drug Administration. (n.d.). ChooseMyPlate – Protein group. https://www.choosemyplate.gov/eathealthy/protein-foods
- Mayo Clinic. (2019, July 19). Vegetarian diet: How to get the best nutrition. https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
- UNICEF. (2018, September 10). Global hunger continues to rise. https://www.unicef.org/press-releases/global-hunger-continues-rise-new-un-report-says
- UNICEF. (2015, December 23). Severe acute malnutrition. https://www.unicef.org/nutrition/index_sam.html
- Nutriset Group. (n.d.). Plumpy’Nut. https://www.nutriset.fr/products/en/plumpy-nut
- Baum, J. I., Kim, I-Y., & Wolfe, R. R. (2016). Protein consumption and the elderly. Nutrients, 8(6), 359. https://dx.doi.org/10.3390%2Fnu8060359
- Montero-Fernández, N., & Serra-Rexach, J. A. (2013). Role of exercise on sarcopenia in the elderly. European journal of physical and rehabilitation medicine, 49(1), 131-143.
- Chan, D. S. M., Lau, R., Aune, D., Vieira, R., Greenwood, D. C., Kampman, E., & Norat, T. (2011). Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE, 6(6), e20456. https://doi.org/10.1371/journal.pone.0020456
- Sinha, R., Cross, A. J., Graubard, B. I., Leitzmann, M. F., & Schatzkin, A. (2009). Meat intake and mortality: A prospective study of over half a million people. Archives of Internal Medicine, 169(6), 562-571. https://dx.doi.org/10.1001%2Farchinternmed.2009.6
- Kuck, G., and Schnitky, G. (2021, May 12). An overview of meat consumption in the United States. Department of Agriculture and Consumer Economics, University of Illinois at Urbana-Champaign. farmdoc daily, 11, 76. https://farmdocdaily.illinois.edu/2021/05/an-overview-of-meat-consumption-in-the-United-States.html
- Gardner, C. D., Hartle, J. C., Garrett, R. D., Offringa, L. C., & Wasserman, A. S. (2019, April). Maximizing the intersection of human health and the health of the environment with regard to the amount of type of protein produced and consumed in the United States. Nutrition Review, 77(4), 197-215. Doi:10.1093/nutrit/nuy073.
- National Institutes of Health. National Institute of Allergy and Infectious Diseases. (2018, October 29). Food allergy – Overview. https://www.niaid.nih.gov/diseases-conditions/food-allergy
- National Institutes of Health. National Institute of Allergy and Infectious Diseases. (2018, October 25). Food allergy – Characterizing food allergy & addressing related disorders. https://www.niaid.nih.gov/diseases-conditions/food-allergy-characterizing
- American Academy of Allergy, Asthma, & Immunology. (2020, September 28). The myth of IgG food panel testing. https://www.aaaai.org/tools-for-the-public/conditions-library/allergies/igg-food-test
- Cornell, L. (2022, April 23). Food allergy and sensitivity testing. 2022 Texas Academy of Nutrition and Dietetics Annual Conference and Exhibition, San Antonio, TX, United States.
building blocks of protein
Nine amino acids that must be consumed in the diet because the body cannot make them in sufficient amounts to sustain life
Amino acids that can be made by the body in sufficient amounts so do not need to be consumed in the diet.
Nonessential amino acids that become essential during specific times in the lifecycle or any time due to diseases or conditions.
The creation of proteins in body cells by combining amino acids is specific sequences.
First step of protein synthesis where the gene recipe for a protein is copied onto messenger RNA (mRNA)
Organelle of a cell where protein synthesis occurs
Second step of protein synthesis where the gene-coded recipe for a protein on the messenger RNA is followed and amino acids are combined in a specific order to make a unique protein. Translation occurs in the ribosome of a cell.
Process of combining molecules by removing a water molecule. This occurs in many instances in the body including the building of macromolecules such as glycogen, storage fat, and protein.
bonds linking amino acids together
Protein consisting of four or more (many) amino acids
physical changes such as unraveling that occur in proteins when they are exposed to heat, acid, high salt concentrations, alcohol, and mechanical agitation
The process of chemically splitting macromolecules by the addition of water. It is used in many places in the body, including digestion where large carbohydrates, lipids, and proteins are split into smaller molecules.
scientific word for chewing
muscle contractions of the digestive tract, squeezing from top to bottom
muscular contractions of the digestive tract squeezing from the sides
sphincter between the esophagus and the stomach
an inactive precursor to an active or functional enzyme
First active protein-digesting enzyme, found in the stomach. Pepsin begins the chemical breakdown of proteins into smaller units.
partially digested food that is a semiliquid mass that leaves the stomach about a tablespoon at a time and enters the duodenum of the small intestine
sphincter between the stomach and the small intestine
First and smallest section of the small intestine, where the bulk of chemical digestion of foods occurs.
hormone released from the duodenum that initiates release of pancreatic juices containing macronutrient-digesting enzymes and bicarbonate for neutralizing hydrochloric acid from the stomach
hormone released from the pancreas that tells the gallbladder to release bile
Enzymes that chemically digest proteins
Enzymes released from the intestinal villi that chemically digest tri- and di-peptides, splitting them into individual amino acids
Second and longest section of the small intestine where chemical digestion and absorption of nutrients occurs
Third and final section of the small intestine where much nutrient absorption occurs.
Form of nutrient absorption that requires both a carrier and energy in the form of ATP. This means that nutrients can be absorbed even if there are already many in the blood (against a concentration gradient)
transformation process that changes one amino acid into another by modifying the R group
Formation of glucose from non-carbohydrate sources such as lipids and protein
The process that forms triglycerides from glycerol and fatty acids.
Body processes of protein breakdown and protein synthesis
Amino acids from ingested proteins plus dismantled proteins that can be used by the body cells for protein synthesis.
proteins that catalyze specific chemical reactions by lowering the amount of energy and time it takes for the reaction to occur
chemical messengers produced by endocrine glands such as the thyroid, pituitary, adrenal glands and the brain's hypothalamus and secreted directly into the blood
Most abundant protein in the blood; important for fluid balance between blood and body cells
Measure of how acidic or basic a substance is. A low pH indicates more acidic, a pH of 7.0 is considered neutral, and a high pH is more basic.
Protein on red blood cells that transports oxygen
Transport mechanism requiring a protein carrier which shuttles molecules such as some nutrients across cell membranes. The process does not require energy so is considered passive.
Blood protein produced in response to a specific foreign invader or antigen. Antibodies combine with "alien" substances such as bacteria or viruses, and counteract their ability to infect body cells
breakdown of molecules into smaller units
The balance between nitrogen consumed through protein intake and nitrogen losses in urine, feces, hair, nails and skin.
Amount of nitrogen excreted is greater than the amount ingested through protein intake. To compensate the body must break down proteins to meet cellular requirements. Occurs primarily when a person is diseased or is consuming a low protein diet.
Occurs when nitrogen intake via protein consumption is greater than nitrogen excretion. This occurs during times of child growth, pregnancy, and wound healing.
Food that contain fewer than nine of the essential amino acids
Foods that contain all nine essential amino acids in relatively high amounts
two or more incomplete protein foods that, when consumed together, provide all nine of the essential amino acids
The amino acids in the shortest supply relative to its requirement
Form of malnutrition that occurs when calorie intake is adequate but protein intake is lacking. It primarily affects children.
Form of malnutrition that occurs when both calories and protein intake are inadequate. It affects both children and adults.
A dangerous combination of low protein intake and low calorie intake leading to malnutrition.
reduction of muscle mass in the body; often accompanies aging
an immune response to a protein in a food product, the response can involve any area of the body: mouth, tongue, skin, GI tract, lungs, cardiovascular system, and can even cause death
a negative response to a food product involving primarily the gastrointestinal system (gas, bloating, cramps, diarrhea)
