21
Case
Age: 27-year-old woman
Reason for referral to ophthalmology: Binocular diplopia and intermittent ptosis
Past medical history: None
Past ocular history: None
Medications: None
Habits: Non-smoker and does not drink alcohol
HPI: Two weeks ago, she noticed that her left eyelid drooped at the end of the day and this resolved when she woke up in the morning. This occurred on two occasions. One week ago, she developed double vision when looking to the right. Objects were separated horizontally and this resolved if she covered either eye. Due to her double vision, she presented to the emergency room and a CT scan of the head without contrast was normal. A neuro-ophthalmology consultation was requested.
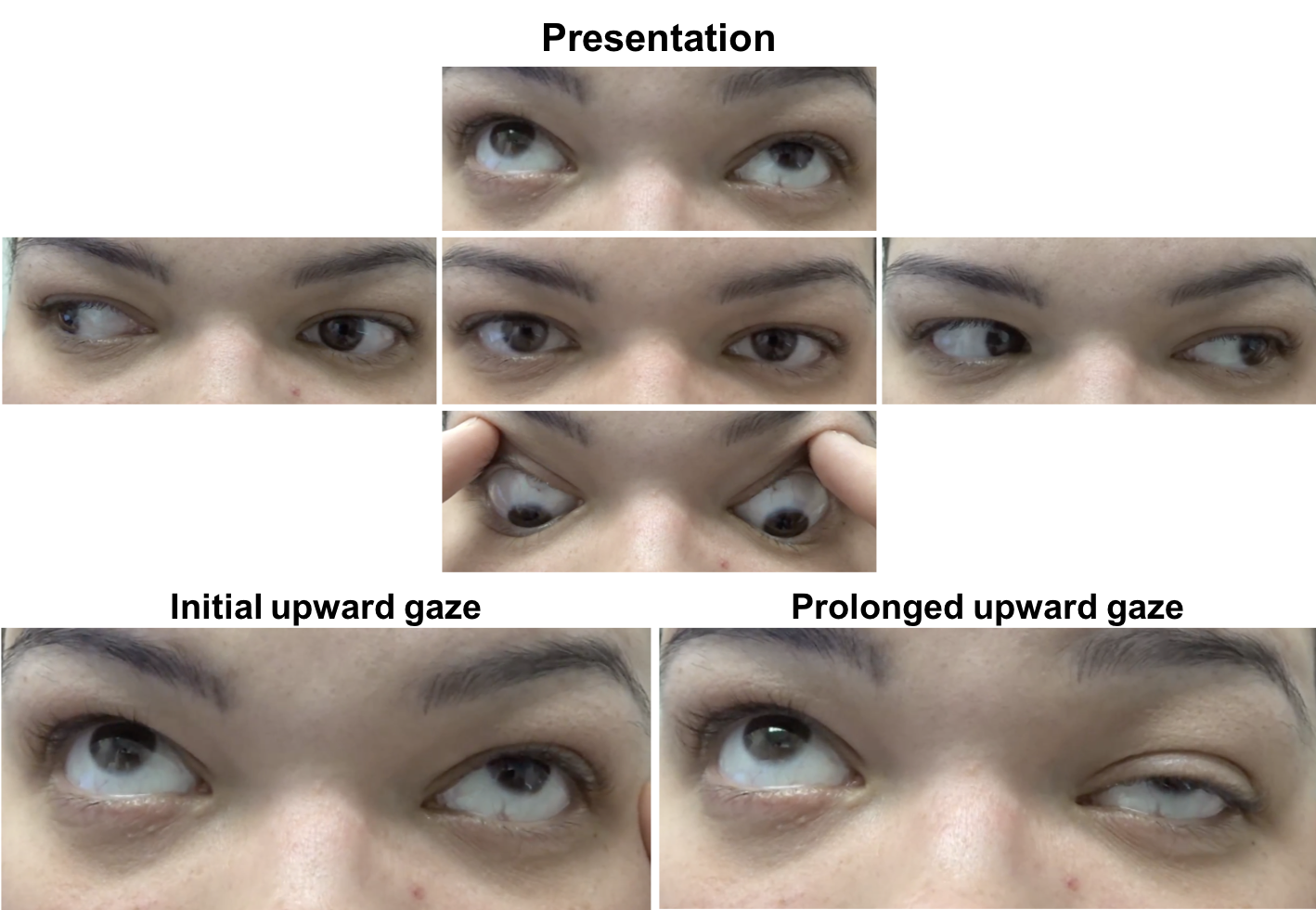
Ophthalmological examination:
Blood pressure: 117/78, heart rate 80
Visual acuity: 20/20 OD, 20/20 OS
Pupils are equal sizes and reactive to light, there is no RAPD
Color vision: 14/14 correct Ishihara plates in both eyes
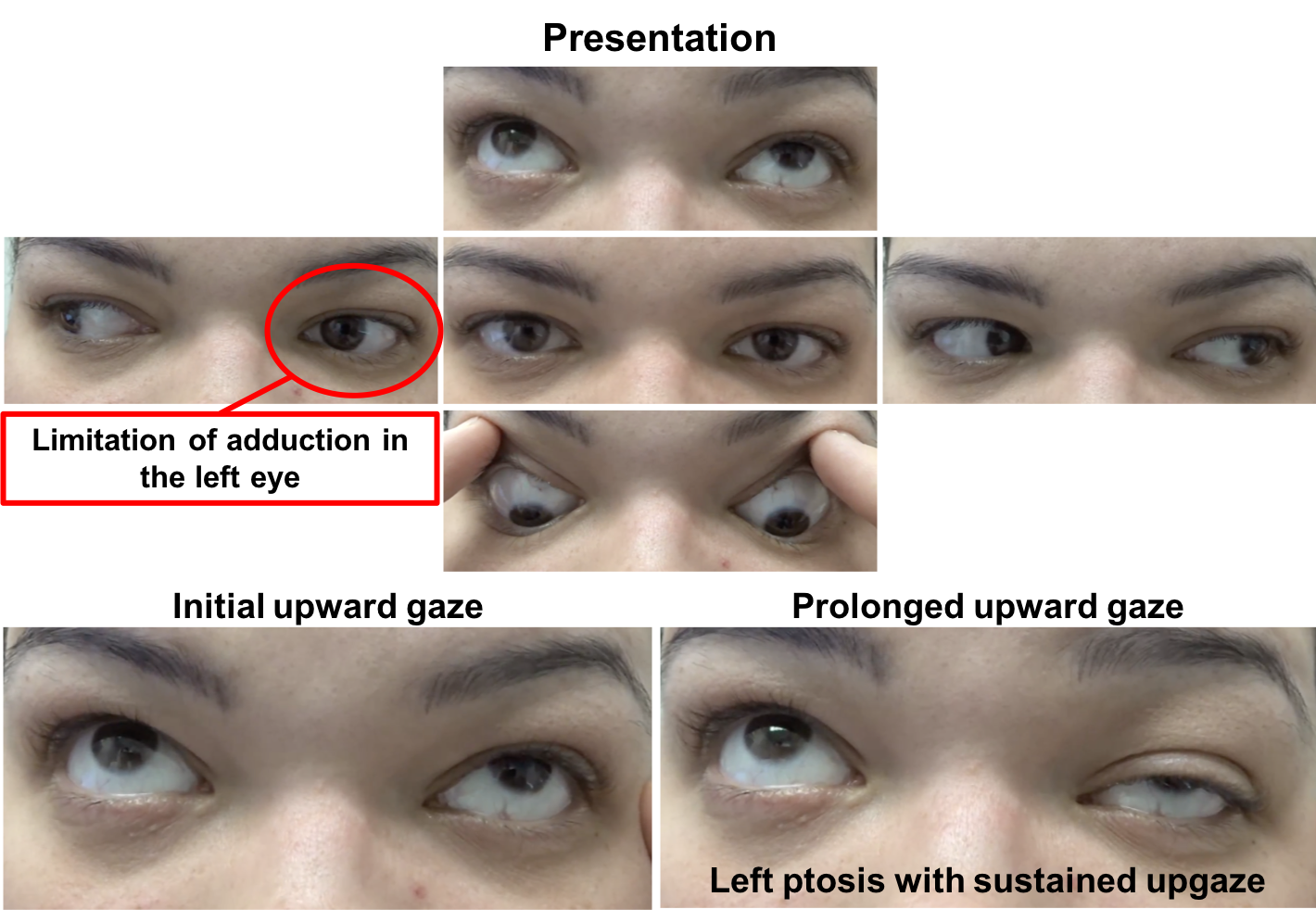
External examination and ocular motility and alignment are shown below
Slit lamp examination is normal
Neurological examination is otherwise normal
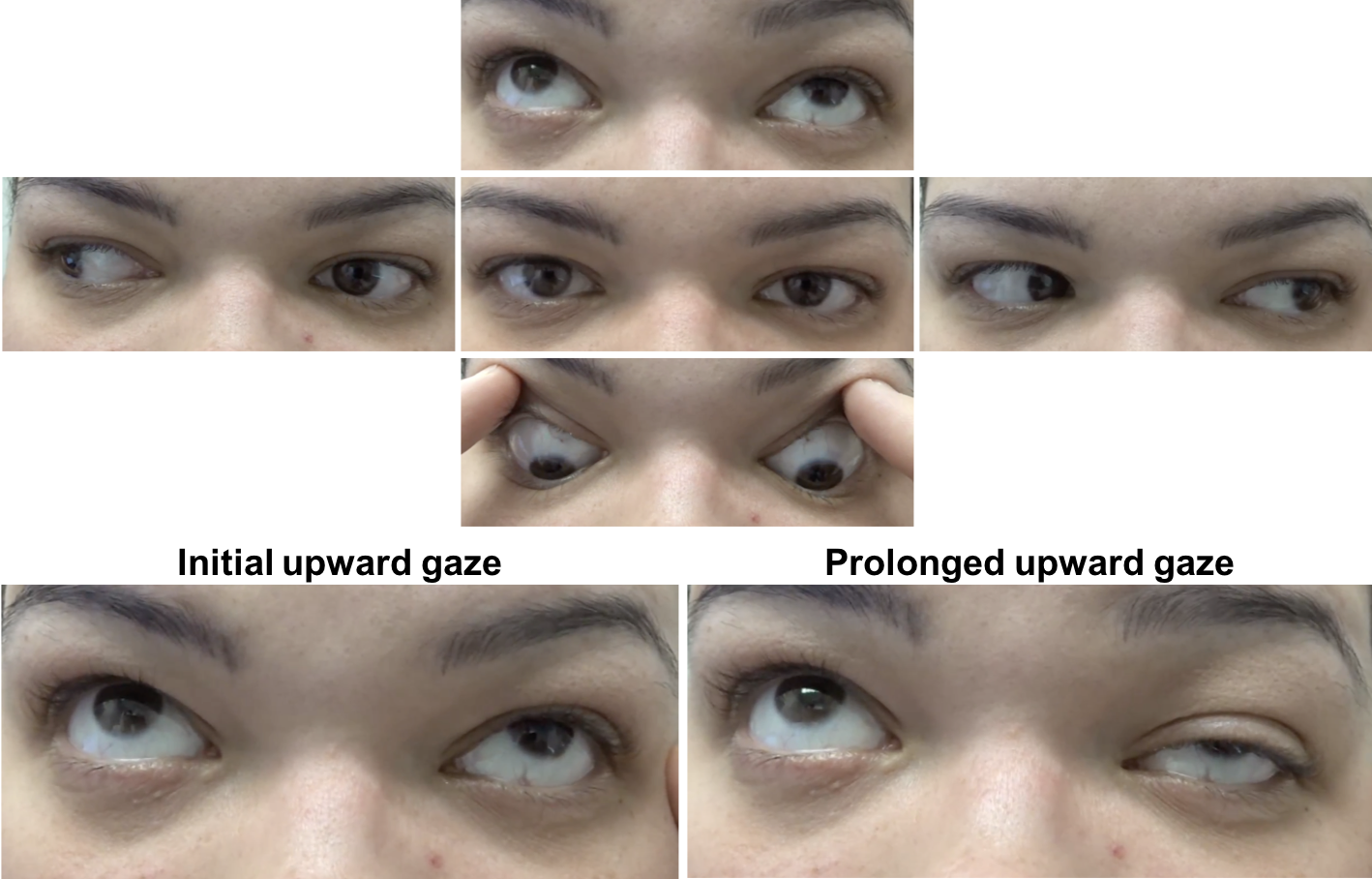
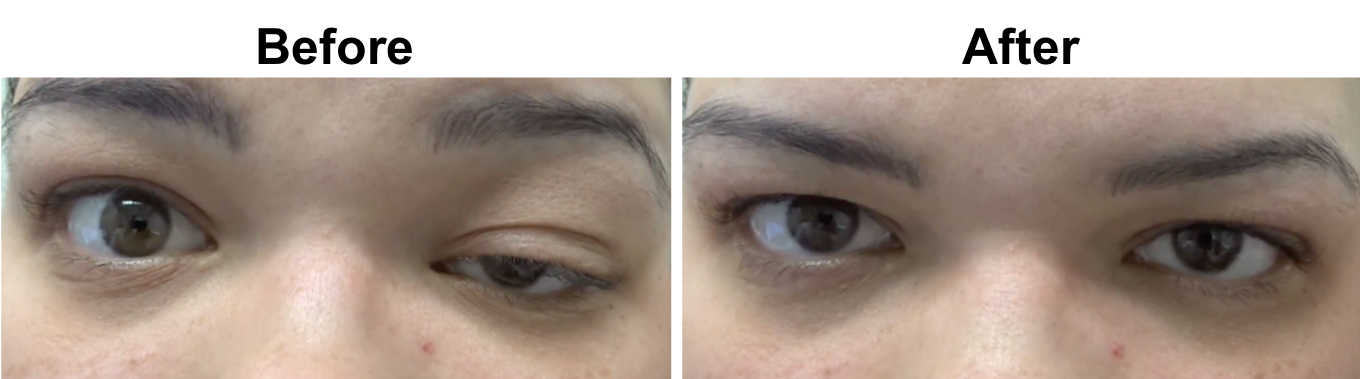
1. Which of the following may have resulted in the following change?
- Sleep
- Ice
- Edrophonium
- Neostigmine
- All of the above
1. Which of the following may have resulted in the following change? 5. All of the above
The patient has fluctuating ptosis and double vision, which is highly suggestive of ocular myasthenia gravis (OMG). In the picture above there is improvement in the left ptosis in the “after” photo. A number of clinical tests may be used to help in the diagnosis of OMG including the sleep test, ice test, and pharmacological testing. The sleep test involves having the patient rest with their eyes closed for approximately 30 minutes and immediately re-evaluating the eyelids and ocular motility. The improvement typically lasts 2 to 5 minutes after which the ptosis and ophthalmoparesis recur. This is a safe and moderately sensitive and specific way to evaluate for OMG. Another clinical test in patients with ptosis suspected of OMG is the ice test. This involves placing ice on the involved eyelid for at least 2 minutes after documentation of the size of the palpebral fissure. The size of the palpebral fissure is then recorded and compared to the pre-ice measurement. The test is considered positive when there is an increase in the palpebral fissure compared to the pre-ice measurement, since the temperature change in the skeletal muscle fibers inhibits the activity of acetylcholinesterases.
Pharmarcological testing may also be used in the diagnosis of OMG. Edrophonium chloride testing (“Tensilon test”) is particularly useful for patients with ptosis since it has a rapid onset of action (30 seconds) and short duration (5 minutes) of effect. Once the position of the eyelids has been documented, 1-2 mg of edrophonium is injected and the patient is observed carefully for an improvement of the ptosis. If improvement occurs, the test is complete and considered positive. If there is no improvement, an additional 1-2 mg of edrophonium is administered and then a bolus of 5 mg can then be administered. Although the test is most useful in patients with ptosis, a Maddox rod can be used while administering the medication and a change in ocular alignment determined by asking the patient about the movement of the red line and white light closer to each other. Since edrophonium is a peripheral anticholinesterase agent, it is important to have atropine available during the test in case bradycardia or syncope develops. Neostigmine is a similar antichoinesterase agent, but has a longer duration of action, which allows for quantitative measurements of prism-cover testing to be repeated. It is typically administered by mixing 0.6 mg of atropine sulfate with 0.5-1.5 mg of neostigmine in a 3 cc syringe delivered via an intramuscular injection.
Clinical Pearl
The sleep or ice test can be used in the clinic to support a diagnosis of myasthenia gravis in a patient with ptosis.
2. After sustained downgaze and a vertical saccade back to primary position, the eyelids briefly elevate before returning to their previous position (see video at https://youtu.be/NsKensaXZ4U). What is this sign called?
- Aberrant regeneration
- Cogan’s lid twitch
- Reverse ptosis
- Lid lag
2.After sustained downgaze and a vertical saccade back to primary position, the eyelids have a brief elevation before returning to their previous position (see video at https://youtu.be/gzs7aTDRYj8). What is this sign called? 2. Cogan’s lid twitch
Cogan’s lid twitch can be seen in patients with OMG. This can be elicited by having the patient look down for 10-20 seconds and then redirecting their eyes back to primary position with a quick vertical saccade. A definite overshoot or twitch of the upper eyelid soon after the patient has returned to primary gaze constitutes a positive finding. It is thought to be due to rapid recovery and easy fatigability of the myasthenic muscle. Patients with OMG may also have “hopping lid twitches” seen with horizontal gaze.
Clinical Pearl
Patients with ocular myasthenia gravis may have “hopping lid twitches” on horizontal gaze and a Cogan’s lid twitch.
3. What other examination finding supports the diagnosis of myasthenia gravis?
- Weakness of the orbicularis oculii muscles
- Sustained normal strength of the orbicularis muscle since it is the levator muscle that is usually affected
- Fluttering of the eyelids
- Erythema of the eyelid skin
3. What other examination finding supports the diagnosis of myasthenia gravis? 1. Weakness of the orbicularis oculii muscles
Orbicularis strength should be tested in all patients suspected of OMG since it is commonly decreased. Orbicularis weakness is a very sensitive clinical sign. During sustained forceful closure of the eyelids, OMG patients may develop partial opening of the palpebral fissure, which is called the “peek sign”. This patient had bilateral orbicularis weakness and her eyelids could easily be opened despite forceful eyelid closure.
4. What should detailed examination of the pupils reveal?
- Larger pupil diameter after sustained upgaze
- Larger pupil diameter after prolonged stimulation with light
- Anisocoria with the larger pupil on the size with more ptosis
- Equal pupil sizes
4. What should detailed examination of the pupils reveal? 4. Equal pupil sizes
Ocular myasthenia gravis should be considered only in cases of pupil-sparing ophthalmoplegia with or without ptosis since it only affects skeletal and not visceral musculature. Pupillary dilation is not controlled by skeletal muscle and the pupils would therefore not be expected to be affected in this condition.
5. What other testing can help confirm the diagnosis in this patient?
- MRI of the brain and spine
- Serology
- Lumbar puncture
- None of the above
5. What other testing can help confirm the diagnosis in this patient? 2. Serology
Serologic and electrophysiologic testing can be used to confirm the diagnosis of OMG. The pathophysiology of OMG involves antibodies directed against acetylecholine receptors at the post-synaptic neuromuscular junction. Anti-acetylcholine receptor antibodies (binding, blocking and modulating antibodies) can be detected in the serum in more than 85% of patients with generalized myasthenia gravis, but the sensitivity is between 45 and 60% in patients with OMG. This is a very specific test with false positives being rare. Repetitive nerve stimulation and single-fiber EMG may also provide confirmation of the diagnosis. The sensitivity of single-fiber EMG is between 60 and 100% and evaluation of the orbicularis oculi and superior rectus-levator complex increases the sensitivity to over 95% in patients with OMG in the hands of an experienced operator.
Clinical Pearl
Acetylcholine receptor binding antibodies are very specific for the diagnosis of myasthenia gravis (rarely have false positives).
Results of serologic testing:
Acetylcholine receptor binding antibody 0.09 mmol/L (normal < 0.02) High
Acetylcholine receptor modulating antibody 92% (normal 0 to 20% loss of AChR) High
6. Which of the following treatments should be considered for this patient’s symptoms?
- Edrophonium
- Atropine
- Pydridostigmine
- Glycopyrrolate
6. Which of the following treatments should be considered for this patient’s symptoms? 3. Pydridostigmine
The initial therapy for patients with OMG is an oral anticholinesterase inhibitor, usually pyridostigmine bromide. It works by slowing down the degradation of acetylcholine that occurs by enzymatic hydrolysis at the neuromuscular junction. A common starting dose of pydridostigmine is 30 mg TID and is titrated up to a maximum dose of 120 mg every 4 hours. It has a rapid onset of action of 15 to 30 minutes and typically lasts for 3 to 4 hours. Due to the cholinergic properties of this medication, patients may experience bothersome side-effects such as abdominal cramping, diarrhea, bronchial secretions, sweating and bradycardia. Side-effects can be minimized by taking the medication with food or with an anticholinergic drug such as glycopyrrolate. This patient had an excellent response to pyridostigmine as shown below.
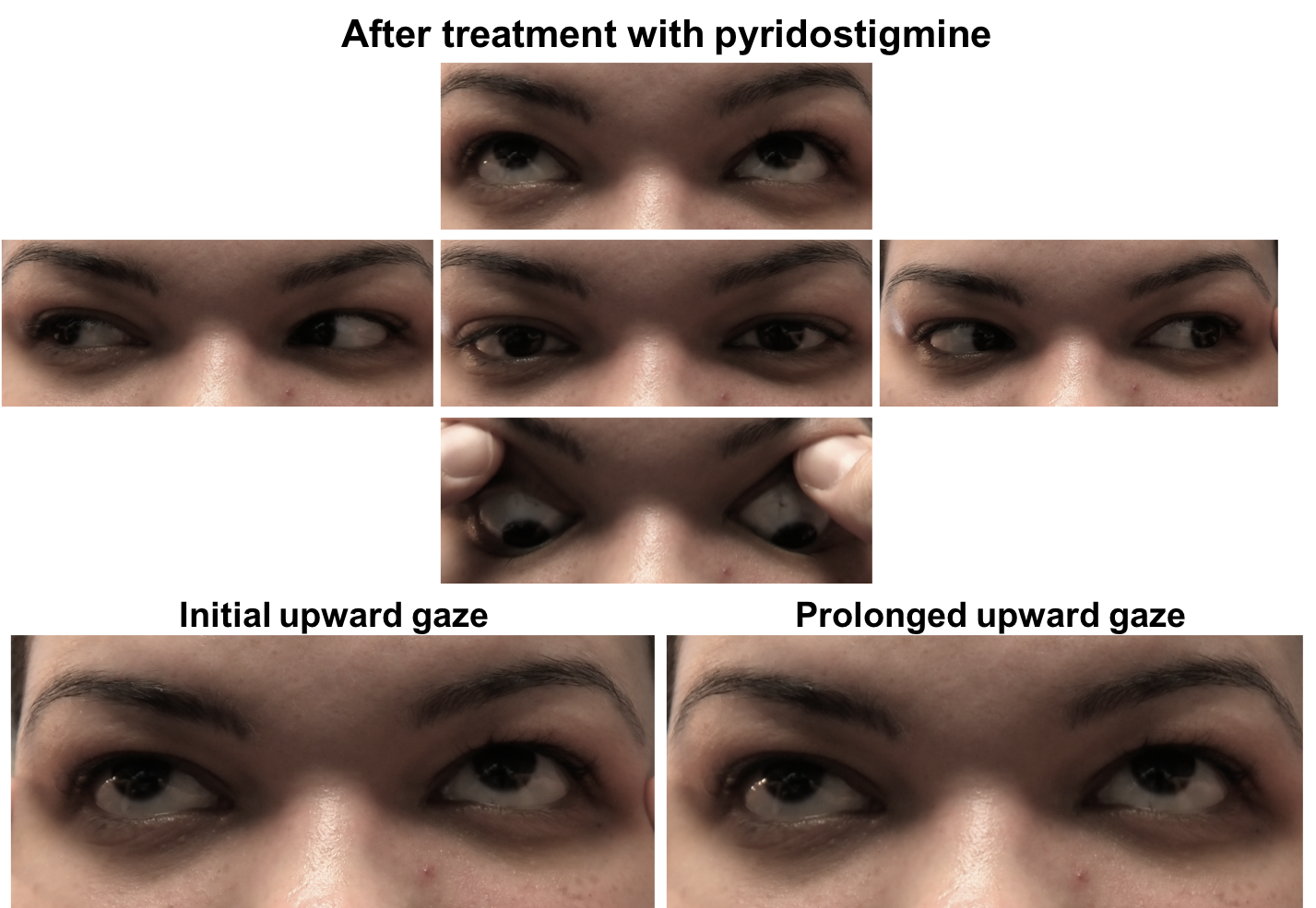
7. What non-pharmacological treatments can be considered in a new patient with double vision from ocular myasthenia gravis?
- Strabismus surgery
- Occlusion of an eyeglass lens
- Ptosis surgery
- Cataract surgery
7. What non-pharmacological treatments can be considered in a new patient with double vision from ocular myasthenia gravis? 2. Occlusion of an eyeglass lens
Simple ways of eliminating diplopia include placing a piece of tape on the lens of a pair of glasses, an eye patch or opaque contact lens. Strabismus or ptosis surgery would not be recommended at this stage due to the fluctuations that are expected to occur with myasthenia gravis.
8. What proportion of patients with ocular myasthenia gravis develop generalized disease?
- None
- One-third
- Two-thirds
- All of the them
8. What proportion of patients with ocular myasthenia gravis develop generalized disease? 3. Two-thirds
Approximately two-thirds of patients presenting with OMG will go on to develop signs and symptoms of generalized disease including extremity weakness and bulbar muscle weakness. One-third of OMG patients will have disease isolated to the extraocular muscles. Most patients (78%) generalize during the first year whereas almost all patients will do so by 3 years (94%).
9. What warning signs should be given to the patient?
- Go immediately to the emergency room if the ptosis gets worse
- Go immediately to the emergency room if you have difficulty breathing
- Go immediately to the emergency room if the diplopia continues
- If you have difficulty breathing decrease the dose of pyridostigmine by half
9. What warning signs should be given to the patient? 2. Go immediately to the emergency room if you have difficulty breathing
Patients with OMG can develop generalized disease and some may have severe bulbar and respiratory muscle weakness necessitating intubation and mechanical ventilation. Rapid therapies such as plasmapheresis and intravenous immunoglobulin are the main therapies of a myasthenic crisis. Chronic immunosuppression with prednisone or other steroid sparing agents are used in patients with generalized disease, but may also be considered for patients with purely ocular symptoms.
10. A patient presents with right ptosis and diplopia and is diagnosed with ocular myasthenia gravis. Why is there lid retraction in the left eye?
- Anatomical variation
- Previous ptosis surgery
- Use of prednisone
- Hering’s law
10. A patient presents with right ptosis and diplopia and is diagnosed with ocular myasthenia gravis. Why is there lid retraction in the left eye? 4. Hering’s law
Hering’s law of equal innervation states that equal and simultaneous innervation flows from the brain to yoke muscles including both levator muscles. Since the right eyelid is ptotic, there is increased innervation to that eye in an attempt to elevate it further. Due to the law of equal innervation, the left eyelid receives a similar increase in signal, resulting in eyelid retraction. This can be tested by manually elevating the ptotic right eyelid and observing the left eyelid decrease to its normal position. Other reasons for patients with OMG to have eyelid retraction includes the simultaneous presence of thyroid eye disease or Cogan’s lid twitch.
11. Which of the following is true regarding the epidemiology of myasthenia gravis?
- It usually affects young women of childbearing age
- There is a bimodal distribution with an early peak in young men and a later peak in older women
- There is a bimodal distribution with an early peak in young women and a later peak in older men
- It usually affects young men between 30 and 40 years of age
11. Which of the following is true regarding the epidemiology of myasthenia gravis? 3. There is a bimodal distribution with an early peak in young women and a later peak in older men
Myasthenia gravis can occur at any age, but there is a bimodal distribution to the age of onset with a peak in the second and third decades with a female predominance and a late peak in the sixth to eighth decade with a male predominance. The prevalence of myasthenia gravis has been increasing and attributed to an aging population, longer life span of affected patients, and better recognition of the condition.
12. A patient previously diagnosed with myasthenia gravis returns for follow-up and has new proptosis in the right eye. The most likely reason for this is:
- Accumulation of acetylcholine receptor antibodies
- Disinsertion of the recti muscles from overuse
- Increased myopia
- Thyroid eye disease
12. A patient previously diagnosed with myasthenia gravis returns for follow-up and has new proptosis in the right eye. The most likely reason for this is: 4. Thyroid eye disease
Patients with myasthenia gravis have a higher incidence of autoimmune conditions compared to the general population. Autoimmune thyroid disease has been reported to occur in 3 to 8 percent of patients with myasthenia and screening for thyroid abnormalities is recommended in the initial evaluation of OMG patients. In this case, the patient likely has the co-existence of thyroid eye disease, which has led to the development of proptosis.
13. A patient presents with binocular horizontal diplopia and a limitation of adduction in one eye. His bloodwork is positive for acetylcholine receptor blocking antibodies and TSH is below the lower limit of normal with a high T4. What is the most likely reason for adduction deficit?
- Thyroid eye disease
- Myasthenia gravis
- Orbital myositis
- None of the above
13. A patient presents with binocular horizontal diplopia and a limitation of adduction in one eye. His bloodwork is positive for acetylcholine receptor blocking antibodies and TSH is below the lower limit of normal with a high T4. What is the most likely reason for adduction deficit? 2. Myasthenia gravis
Although myasthenia gravis typically involves more than one extraocular muscle, it has a predilection for the muscles innervated by the 3rd cranial nerve. When a solitary paresis occurs, it commonly results in an adduction deficit, leading to a “pseudo-internuclear ophthalmoplegia”. Thyroid eye disease, in contrast, leads to diplopia by causing thickening and restriction of eye movements. The most commonly involved rectus muscles are the inferior and medial recti (it is very rare for the lateral rectus to be involved in isolation), which would lead to an elevation and abduction deficit. Therefore, ocular myasthenia gravis is more likely to cause an exotropia whereas thyroid eye disease is more likely to cause an esotropia.
Clinical Pearl
Patients with myasthenia gravis are more likely to have an exotropia whereas patients with thyroid eye disease are more likely to have an esotropia.
14. Which of the following is included in the classic triad of Miller Fisher syndrome?
- Ophthalmoplegia
- Ataxia
- Areflexia
- All of the above
14. Which of the following is included in the classic triad of Miller Fisher syndrome? 4. All of the above
Miller Fisher syndrome is an unusual variant of acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome), characterized by the triad of ophthalmoplegia, ataxia, and areflexia, although the triad is not always complete. It should be considered in the differential diagnosis of any patient with multiple cranial neuropathies.
15. How does Miller Fisher syndrome differ from myasthenia gravis?
- Patients with Miller Fisher rarely have ptosis
- Patients with Miller Fisher often have involvement of the pupil
- Patients with Miller Fisher almost always have abnormal MRIs
- Patients with Miller Fisher often have CSF pleocytosis
15. How does Miller Fisher syndrome differ from myasthenia gravis? 1. Patients with Miller Fisher often have involvement of the pupil
Miller Fisher is often considered in the differential diagnosis in cases that appear to involve multiple cranial nerves. However, unlike myasthenia gravis, Miller Fisher involves the pupil in up to 50% of cases (unilateral or bilateral pupillary dilation or poor response to light). Magnetic resonance of the brain with contrast is often normal, but may show enhancement of cranial nerves or abnormal signal in the brainstem or cerebellum.
16. What CSF findings are expected in patients with Guillain-Barré syndrome?
- Pleocytosis (elevated white blood cell count) and elevated protein
- Pleocytosis and normal protein
- Normal cell count and elevated protein
- Normal cell count and protein
16. What CSF findings are expected in patients with Guillain-Barré syndrome? 3. Normal cell count and elevated protein
Cerebrospinal fluid typically shows elevated protein without pleocytosis, which is referred to as albuminocytologic dissociation. Normal protein levels do not rule out the diagnosis since one-third to one-half of patients have normal protein, especially if the CSF is tested within one week of symptom onset. The elevated protein is thought to be due to increased permeability of the blood-nerve-barrier at the level of the proximal nerve roots.
17. What diagnostic test is helpful in patients suspected of Miller Fisher syndrome?
- GQ1b
- CT scan of the head
- Erythrocyte sedimentation rate
- Trial of steroids
17. What diagnostic test is helpful in patients suspected of Miller Fisher syndrome? 1. GQ1b
Antecedent infection is very common in Miller Fisher syndrome, usually with respiratory or gastrointestinal symptoms. Campylobacter jejuni is the most commonly identified agent. GQ1b ganglioside is a glycosphingolipid concentrated in the outer leaflet of neuronal membranes, especially in the paranodal regions of the 3rd, 4th, and 6th cranial nerves and dorsal root ganglion. GQ1b ganglioside contains polysaccharides identical to lipopolysaccharide in the outer membranes of bacteria, which may explain the link to infection. Approximately 80-90% of Miller Fisher cases are positive for GQ1B antibodies.
18. Which of the following is included in the care and treatment of patients with Miller Fisher syndrome?
- Close monitoring as an inpatient
- Treatment with IVIG or plasma exchange in severe disease
- Corticosteroids are often avoided
- All of the above
18. Which of the following is included in the care and treatment of patients with Miller Fisher syndrome? 4. All of the above
Although most patients with Miller Fisher syndrome improve spontaneously without treatment, some patients require considerable supportive care. Approximately one-quarter of patients with Miller-Fisher syndrome will develop extremity weakness. Inpatient monitoring is often recommended and treatment with IVIG or plasma exchange is used in severe cases. Corticosteroids are avoided in acute demyelinating inflammatory polyneuropathy since they have not been shown to have any benefit and may cause worsening.
Case Summary
She had new onset transient of ptosis in the left eye that resolved and binocular horizontal diplopia when looking to the right. Examination revealed a limitation of adduction in the left eye and left ptosis with sustained upgaze. The ptosis resolved after 30 minutes of rest, highly suggestive of ocular myasthenia gravis. Acetylcholine receptor antibodies were positive, confirming the diagnosis. She was started on pyridostigmine and had resolution of her symptoms. She was referred for ongoing management to a neuromuscular neurologist. She developed neck weakness, voice changes and proximal muscle weakness 6 months later and was treated with chronic immunosuppression and had improvement in her symptoms.
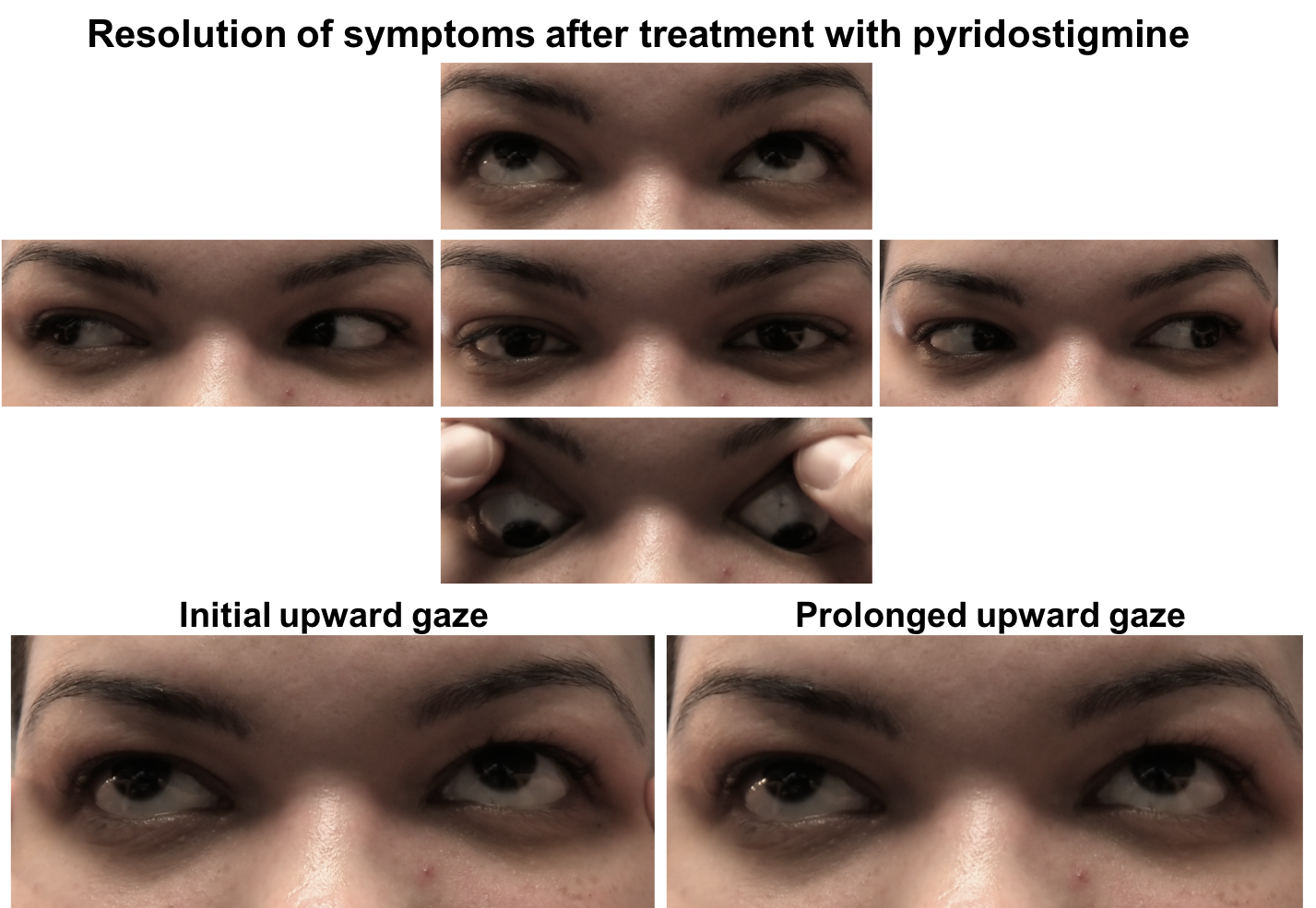
Further reading:
-
Gilhus NE. Myasthenia gravis. New Engl J Med 2016;375:2570-581. http://www.nejm.org/doi/full/10.1056/NEJMra1602678
-
Singman EL, Matta NS, Silbert DI. Use of the Cogan lid twitch to identify myasthenia gravis. J Neuroophthalmol 2011;31:239-240. https://www.ncbi.nlm.nih.gov/pubmed/21654336
-
Pascuzzi RM. The edrophonium test. Semin Neurol 2003;23(1):83-8. https://www.ncbi.nlm.nih.gov/pubmed/12870109
-
Mercelis R, Merckaert V. Diagnostic utility of stimulated single-fiber electromyopgraphy of the orbicularis oculi muscle in patients with suspected ocular myasthenia. Muscle Nerve 2011;43(20):168. https://www.ncbi.nlm.nih.gov/pubmed/21254079
- Smith SV, Lee AG. Update on ocular myasthenia gravis. Neurol Clin 2017;35(1):155-123. https://www.sciencedirect.com/science/article/pii/S0733861916300664