12
Case
Age: 58-year-old white woman
Reason for referral to ophthalmology: New onset blurry vision in both eyes with headache
Past medical history: Hypertension, coronary artery disease, dyslipidema
Past ocular history: None
Medications: Amlodipine, metoprolol, atorvastatin, aspirin
Habits: Non-smoker and does not drink alcohol
HPI: She underwent coronary artery bypass grafting yesterday for coronary artery disease and was noted to have a period of hypotension during the surgery. She was hemodynamically stable in the post-operative unit. When she was transferred to the ward she had a sudden onset severe headache, blurry vision, nausea and vomiting. Her nausea was treated with intravenous medications and an ophthalmology consultation was requested for the blurred vision.
Ophthalmological examination:
Blood pressure: 167/96, heart rate 82
Visual acuity: 20/30 OD, 20/50 OS
Pupils are equal sizes and reactive to light, there is a mild left RAPD
Color vision: 12/14 OD and 6/14 OS correct Ishihara plates
Ocular motility and alignment are normal
Slit lamp examination is normal
Neurological examination is normal
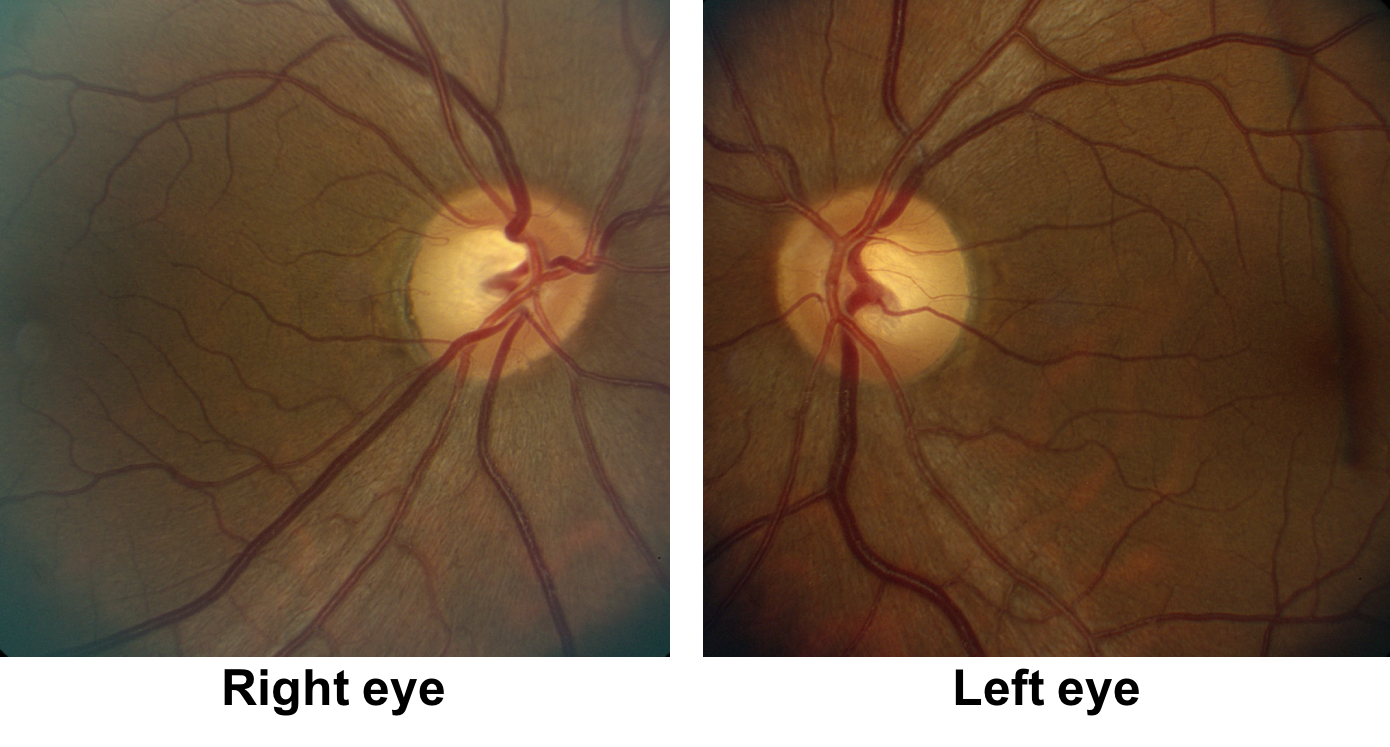
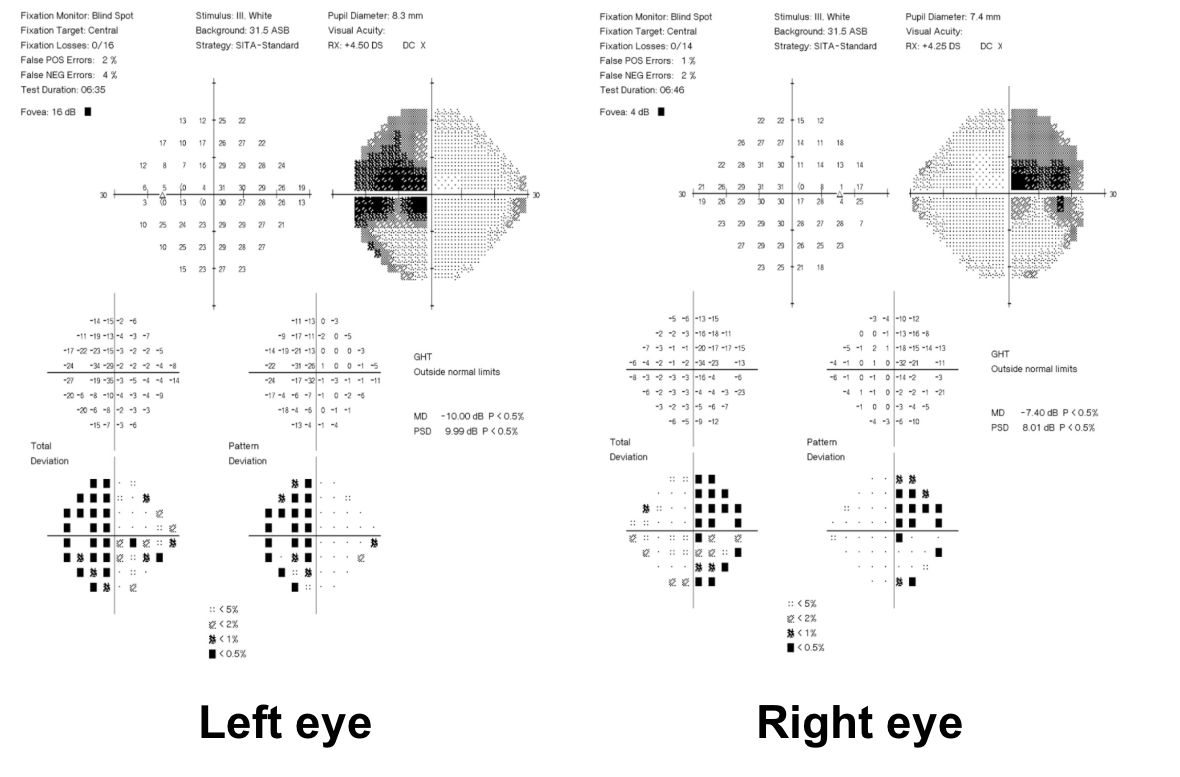
1. What visual field defect does the patient have?
- Enlarged blind spots
- Right incongruous homonymous hemianopia
- Superior greater than inferior bitemporal hemianopia
- Bilateral arcuate scotomas
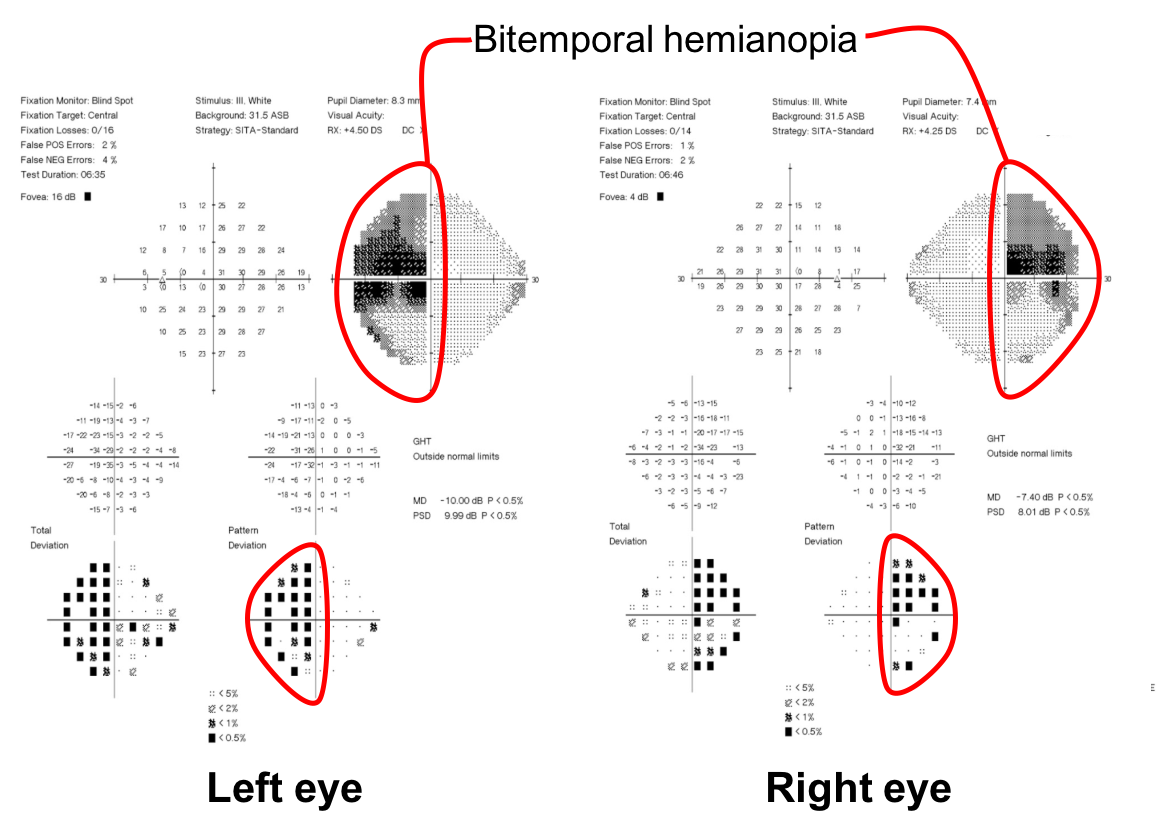
1. What visual field defect does the patient have? 3. Superior greater than inferior bitemporal hemianopia
The patient has a bitemporal hemianopia since the visual field defects are located only in the temporal hemifieds in both eyes. The visual field defect is slightly more dense in the left eye compared to the right eye, consistent with the mild left relative afferent pupillary defect. Bitemporal hemianopias localize to the optic chiasm and indicate that crossing fibers from the nasal retina are affected. Since the visual field defects are more severe superiorly, it suggests that there is compression of the optic chiasm from below.
Clinical Pearl
Mass lesions that compress the optic chiasm such as pituitary adenomas, meningiomas, craniopharyngiomas and aneurysms are the most common causes of bitemporal hemianopia.
2. What is the most likely diagnosis?
- Embolism from a cardiac source to the anterior visual pathways
- Bilateral ischemic optic neuropathies related to hypotension
- Traumatic optic neuropathies related to positioning during surgery
- Pituitary apoplexy
2. What is the most likely diagnosis? 4. Pituitary apoplexy
She has a bitemporal hemianopia in the context of recent cardiac surgery with labile blood pressure and new onset severe headache and nausea. This is very concerning for pituitary apoplexy. Pituitary apoplexy is caused by acute hemorrhage or infarction in a pre-existing pituitary adenoma, which the patient was not aware of in this case. The rapid enlargement of the pituitary tumor compresses adjacent structures and can cause visual field defects and cranial nerve palsies.
3. Which of the following ophthalmologic findings may also be seen in patients with pituitary apoplexy?
- Ptosis
- Relative afferent pupillary defect
- 3rd nerve palsy
- 6th nerve palsy
- All of the above
3. Which of the following ophthalmologic findings may also be seen in patients with pituitary apoplexy? 5. All of the above
Pituitary apoplexy commonly presents with neuro-ophthalmic signs. If the pituitary gland is displaced upwards, the patient is at risk for vision loss from compression of the anterior visual pathways, whereas lateral displacement of the pituitary gland may affect the cranial nerves in the cavernous sinus (cranial nerves 3, 4, V1, V2, and 6). Cranial nerve 3 and 6 are the most commonly involved. Cranial nerve 4 palsy occurs in association with cranial nerve 3 or 6 and is rarely an isolated finding in pituitary apoplexy.
Clinical Pearl
Pituitary apoplexy must be considered in any patient with sudden onset headache and cranial nerve palsy or vision loss. The exact signs and symptoms depends on the direction and degree of expansion of the pituitary adenoma.
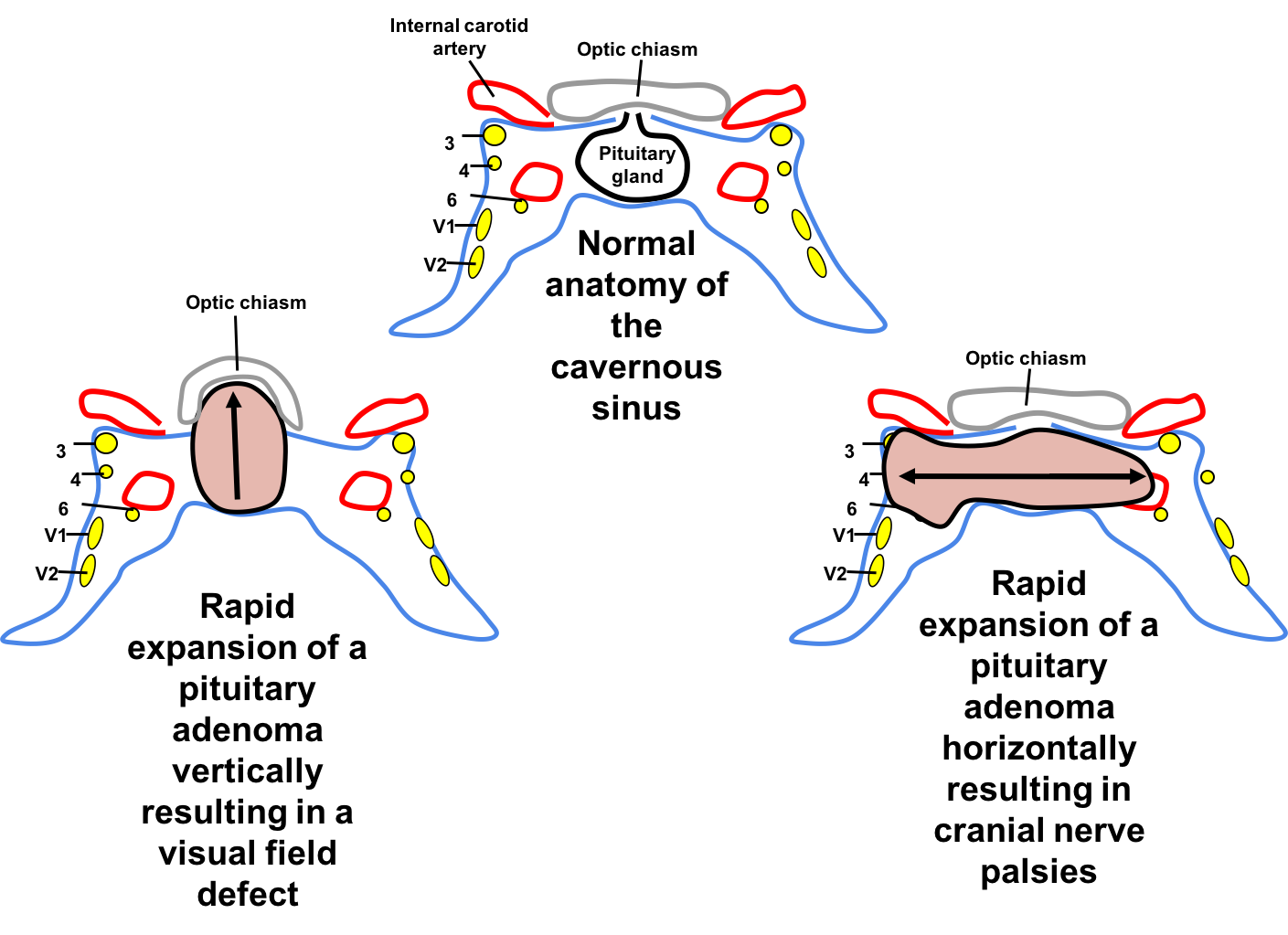
4. What is the next best course of action?
- Outpatient MRI of the brain and orbits with contrast
- Continue cardiac rehabilitation and outpatient neuro-ophthalmology follow-up
- Immediate cardiac ECHO to assess for source of cardiac thrombus
- Immediate neuroimaging and bloodwork to evaluate endocrine function
4. What is the next best course of action? 3. Immediate neuroimaging and bloodwork to evaluate endocrine function
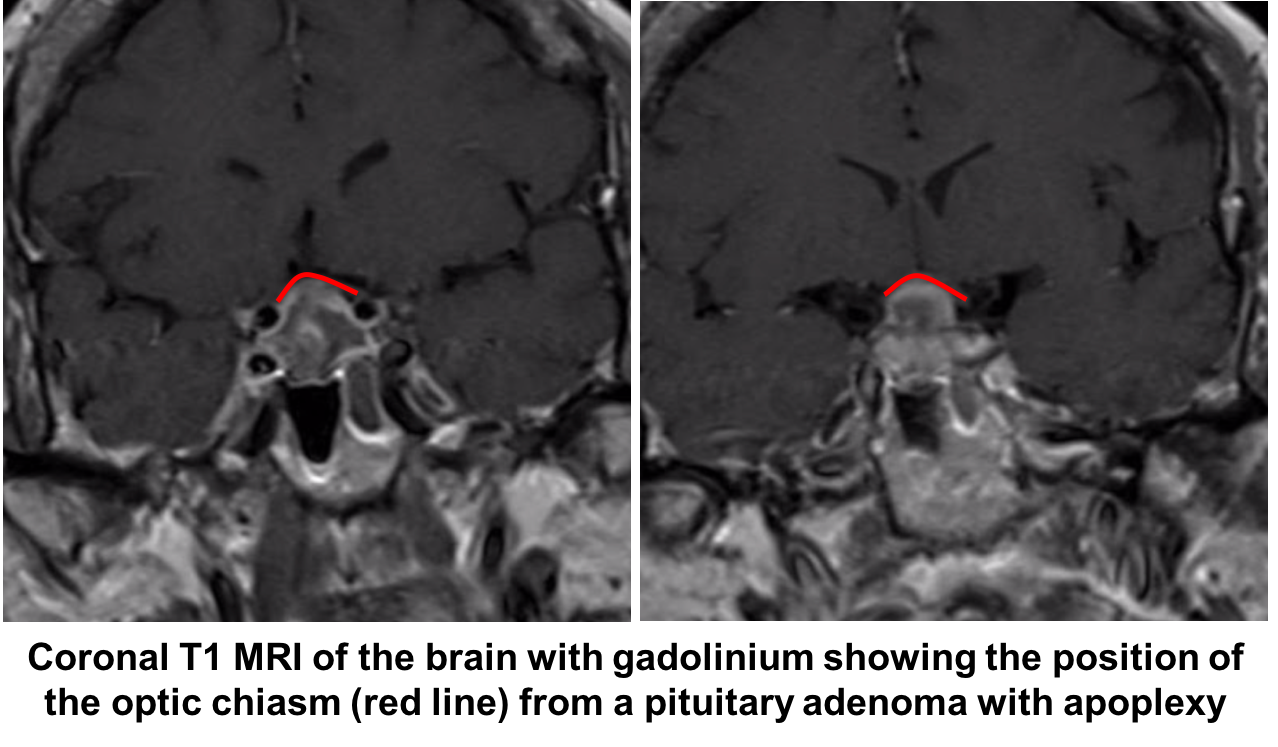
Given the strong suspicion for pituitary apoplexy, the patient requires urgent neuroimaging and MRI is the imaging modality of choice and has been found to confirm the diagnosis in over 90% of patients. A dedicated CT scan can be performed if MRI is contraindicated or not available, but has a lower diagnostic yield. All patients with suspected pituitary apoplexy should have urgent blood samples drawn for a complete blood count, electrolytes, coagulation, glucose, random cortisol, prolactin, thyroid stimulating hormone, T4, growth hormone, luteinizing hormone, follicle stimulating hormone, testosterone in men and estradiol in women.
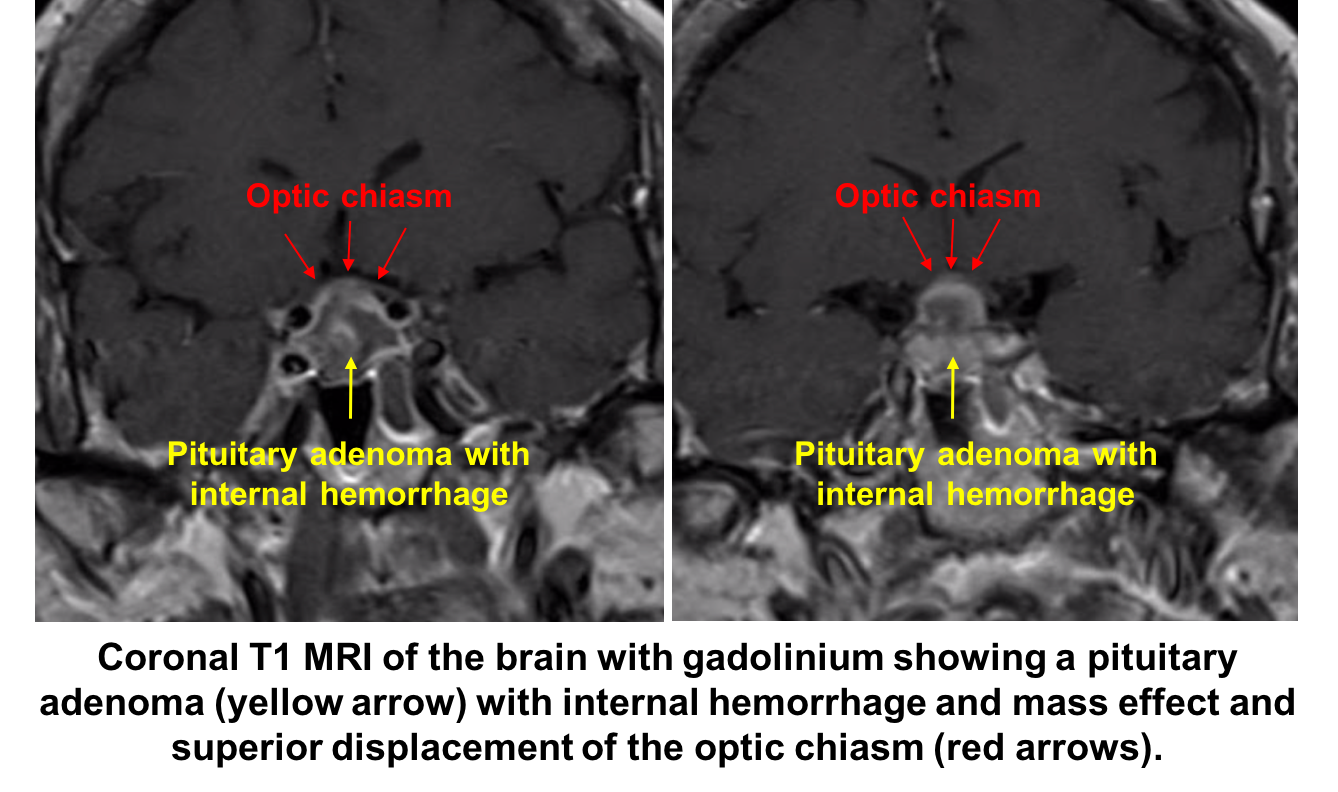
5. When she is transferred back to the ward she becomes hemodynamically unstable, what is the next best step in her care?
- Intravenous hydrocortisone and drawing of blood for baseline endocrine function tests
- Bedside ECHO
- Blood typing and cross-matching
- Hypertonic saline
5. When she is transferred back to the ward she becomes hemodynamically unstable, what is the next best step in her care? 1. Intravenous hydrocortisone and drawing of blood for baseline endocrine function tests
Acute adrenal insufficiency is seen in about two-thirds of patients with pituitary apoplexy and is a life-threatening condition. The patient should be managed by a specialist neurosurgical and endocrinology team to carefully monitor fluid and electrolyte balance, replacement of corticosteroids and supportive measures. Hydrocortisone is usually given as an intravenous bolus of 100-200 mg followed by 2-4 mg per hour by continuous infusion.
The patient was stabilized with intravenous hydrocortisone and thyroxine replacement in the neurosurgical ICU. Neurosurgery and endocrinology were actively involved in her care.
Laboratory workup revealed:
Prolactin 0.5 g/L (Normal 2.8 to 29.9 g/L) Low
Free thyroxine 0.6 g/L (Normal 0.9 to 1.8 g/L) Low
Luteinizing hormone 1.2 IU/L (Normal 2.4 to 15.9 IU/L) Low
Follicle-stimulating hormone 1.4 IU/L (Normal 1 to 14 IU/L)
AM Cortisol 5 g/dL (Normal 5 to 25 g/dL)
Her visual acuity and visual field continued to worsen the next day and she was taken to the operating room for a transphenoidal resection of the pituitary adenoma.
Post-operative neuro-ophthalmology follow-up demonstrated normal visual function.
6. Which of the following are precipitating factors for pituitary apoplexy?
- Hypertension
- Cardiac surgery
- Anticoagulation therapy
- Pregnancy
- All of the above
6. Which of the following are precipitating factors for pituitary apoplexy? 5. All of the above
Hypertension is the most common predisposing factor for pituitary apoplexy. Other precipitating factors include major surgery, particularly coronary artery bypass surgery, dynamic pituitary function tests with GnRH, TRH and CRH, anticoagulation therapy, coagulopathies, estrogen therapy, initiation or withdrawal of dopamine receptor agonists, radiation therapy, pregnancy, and head trauma.
7. Which of the following OCTs of the retinal nerve fiber layer (RNFL) most likely represents a patient with a new bitemporal hemianopia from pituitary apoplexy? Assume the tumor had no contact with the anterior visual pathways before the apoplexy.
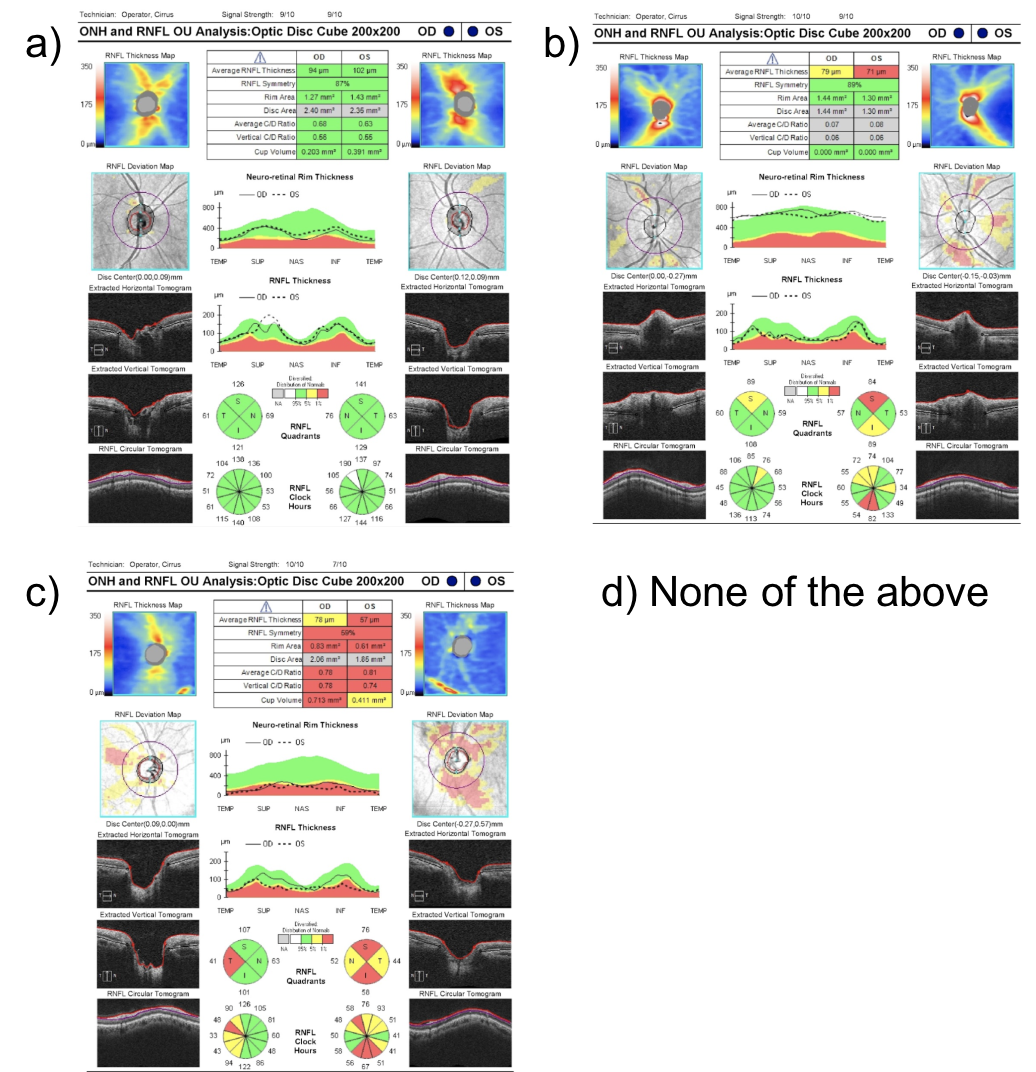
7. Which of the following OCTs of the retinal nerve fiber layer (RNFL) most likely represents a patient with a new bitemporal hemianopia from pituitary apoplexy? Assume the tumor had no contact with the anterior visual pathways before the apoplexy. 1.
A bitemporal hemianopia from pituitary apoplexy is a result of acute compression of the optic chiasm from the expanding mass. Acutely, there is dysfunction of the nerves in the area of the optic chiasm, but thinning of the RNFL has not occurred. This starts to happen weeks after persistent compression. The OCT of the RNFL shown in option A has a normal RNFL thickness and is most representative of what would be seen in this patient. If the compression continues over time, then thinning of the RNFL would be seen as in options B or C.
8. What is the approximate distance from the roof of the sella turcica to the optic chiasm?
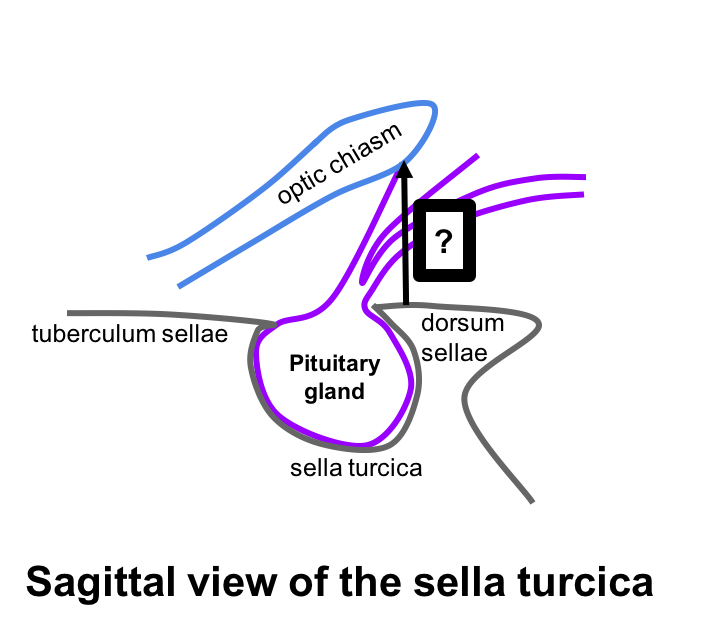
- Less than 5 mm
- 10 mm
- 20 mm
- 50 mm
8. What is the approximate distance from the roof of the sella turcica of to the optic chiasm? 2. 10 mm
The diaphragma sellae is the dural covering of the sella turcica and lies approximately 10 mm below the optic chiasm. This suggests that pituitary tumors should be at least this size to cause mass effect on the optic chiasm.
9. Which of the following sellar lesions is most likely to expand during pregnancy?
- Aneurysm
- Meningioma
- Craniopharyngioma
- Rathke’s cleft cyst
9. Which of the following sellar lesions is most likely to expand during pregnancy? 2. Meningioma
Symptoms from a meningioma may flare during pregnancy and this is thought to be due to the presence of sex hormone receptors on tumor cells, water retention, and engorgement of vessels. The incidence of meningioma in pregnant women is similar to non-pregnant women in the same age group.
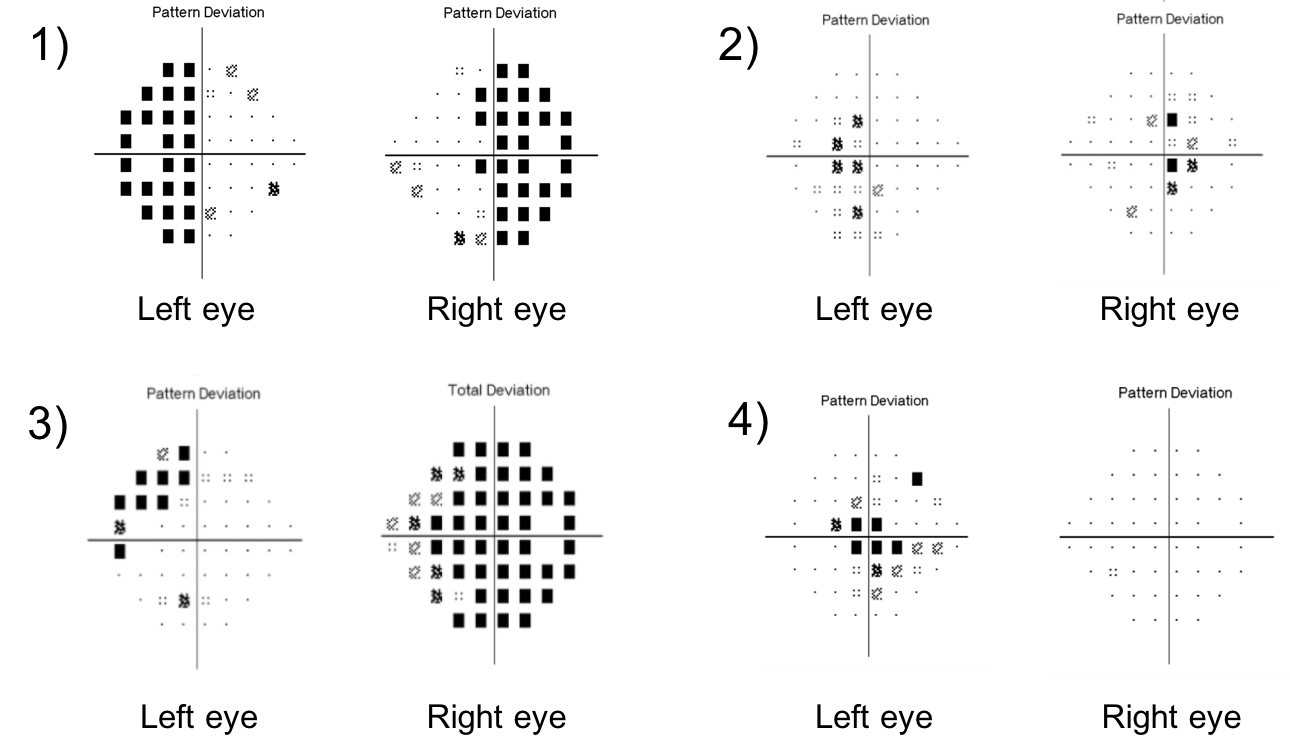
10. Visual fields, T2 coronal MRI images and OCT of the RNFL and ganglion cell analysis are shown below in two patients. Which patient has a better visual prognosis after resection of their pituitary macroadenoma?
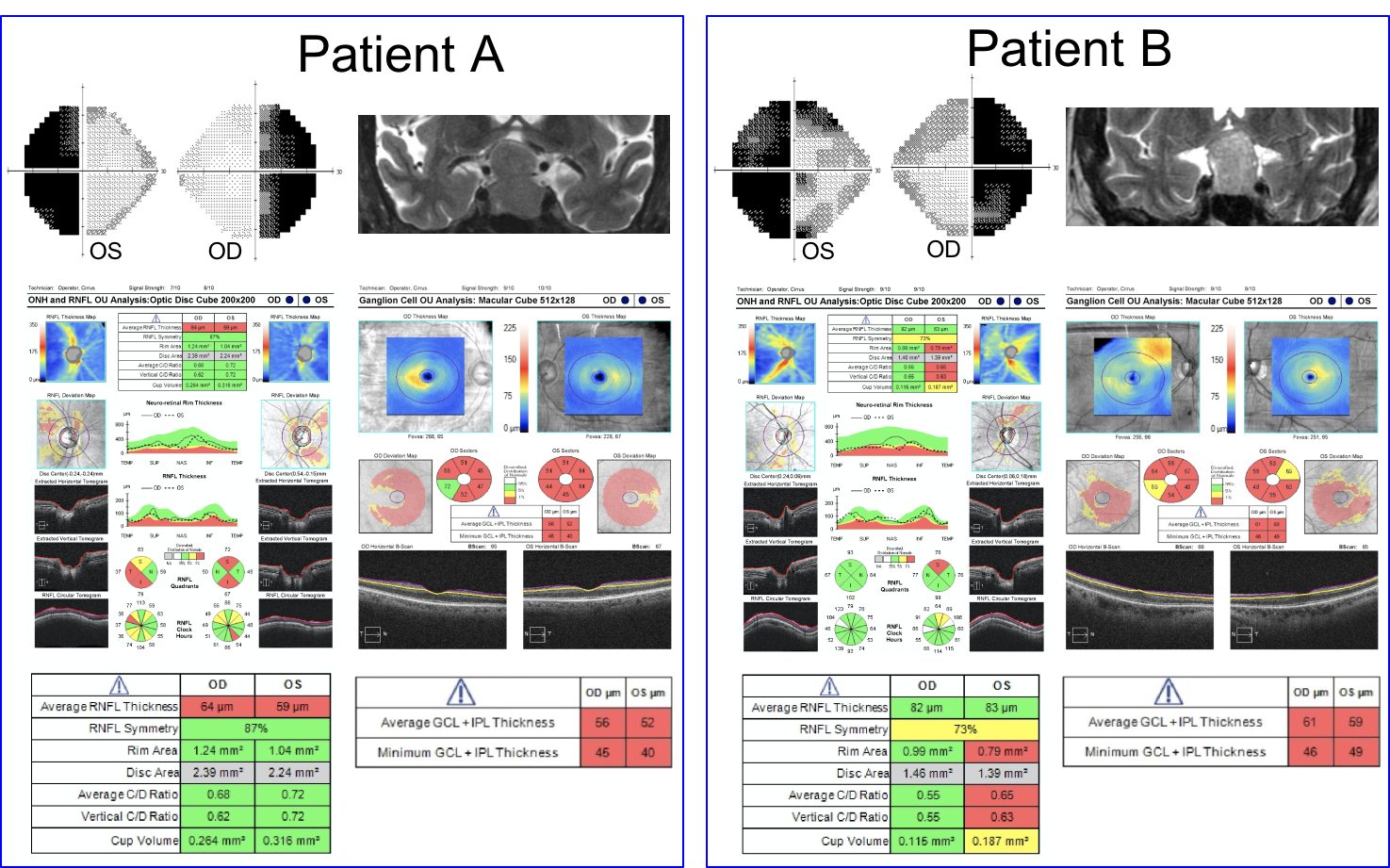
- Patient A since there appears to be less compression of the optic chiasm on the MRI image
- Patient A since there are less changes in the nasal hemifields of both eyes
- Patient B since there the OCT RNFL and macular ganglion cell complex thickness are better preserved
- Patient B since there is less compression of the optic chiasm on the MRI image
10. Visual fields, T2 coronal MRI images and OCT of the RNFL and ganglion cell analysis are shown below in two patients. Which patient has a better visual prognosis after resection of their pituitary macroadenoma? 3. Patient B since there the RNFL and macular ganglion cell complex thickness is better preserved
A number of variables have been studied to predict visual recovery in patients with compression of the optic chiasm. Duration of symptoms, size of the tumor, preoperative visual acuity and visual field, and optic nerve appearance have shown conflicting results in their ability to predict visual outcome. Optic atrophy has been reported to correlate with the degree of visual recovery, but determining the presence and degree of optic pallor is subjective and unreliable. Optical coherence tomography provides an objective means of quantifying the thickness of the RNFL and ganglion cell complex and is recommended by the Congress of Neurological Surgeons as a prognostic tool in patients with visual loss from pituitary tumors. It has been shown that patients with RNFL loss at the time of surgery are less likely to have a return of their visual function after surgery.
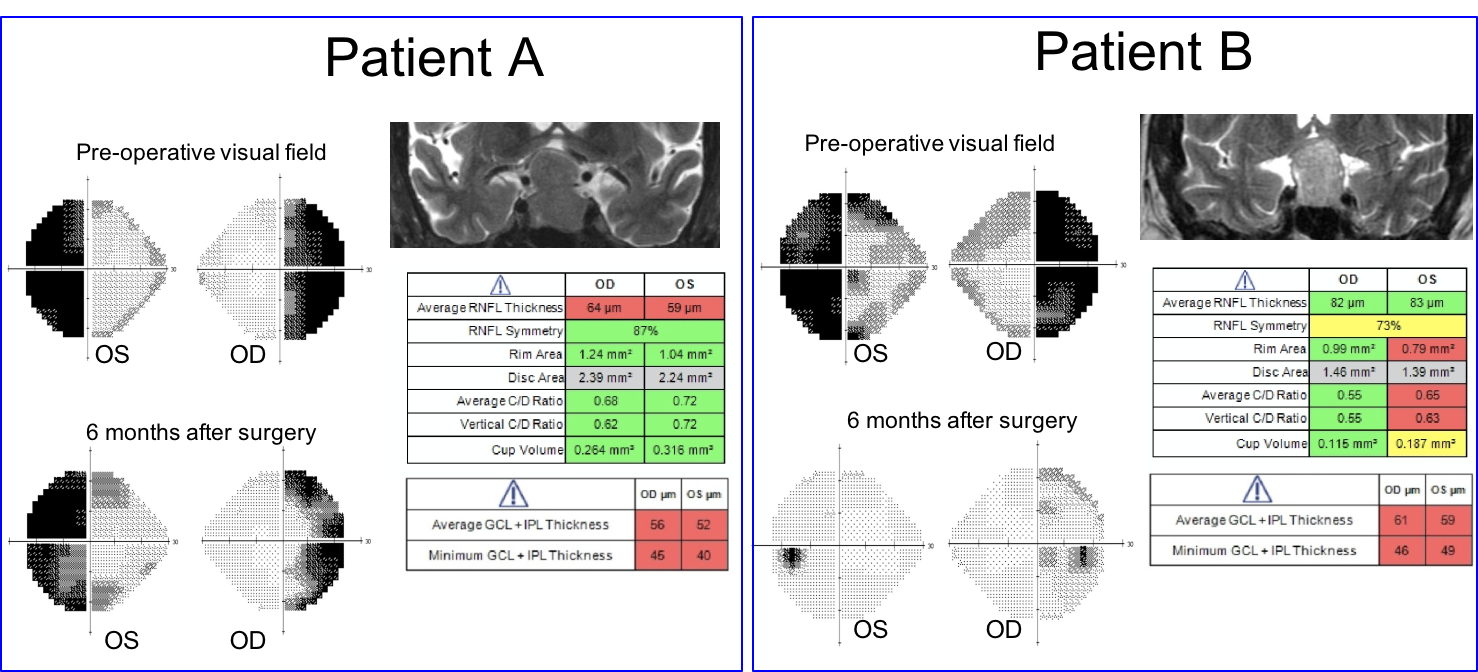
The visual outcome of Patient A and B six months after surgery is shown below.
11. A patient has a sellar cyst with mild mass effect on the optic chiasm as shown below.
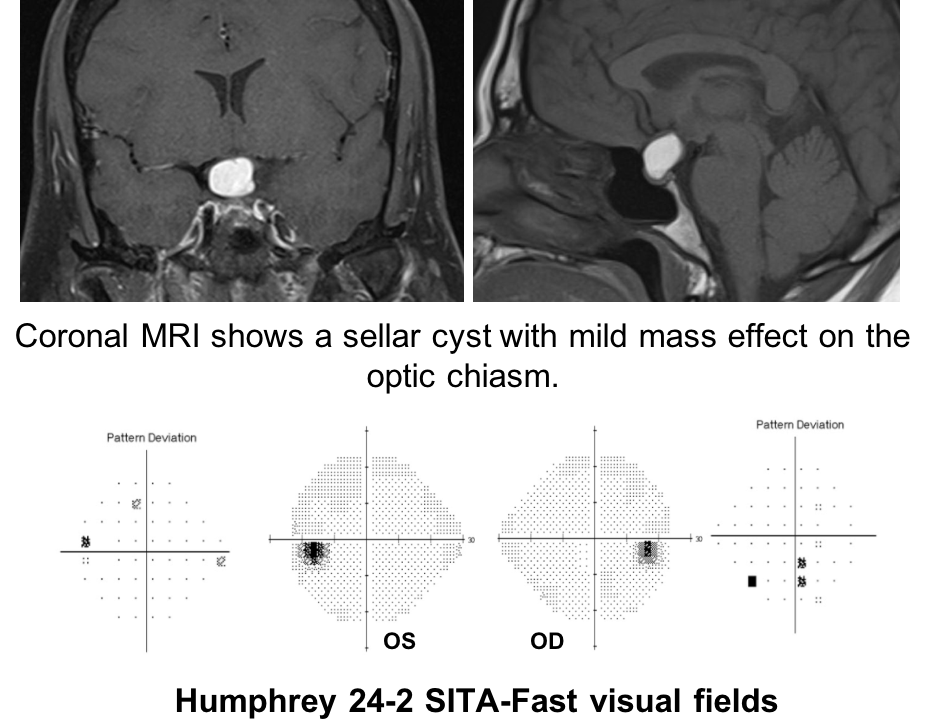 Humphrey 24-2 SITA-Fast visual fields show nonspecific depressed points in both eyes.
Humphrey 24-2 SITA-Fast visual fields show nonspecific depressed points in both eyes.
The patient wants to know if any other test could help determine if the cyst is affecting the anterior visual pathways. What test could best determine this?
- Automated refraction
- Optical coherence tomography
- Multifocal ERG
- No other testing since the visual field is normal
11. A patient has a sellar cyst with mild mass effect on the optic chiasm as shown below. Humphrey 24-2 SITA-Fast visual fields show nonspecific depressed points in both eyes. The patient wants to know if any other test could help determine if the cyst is affecting the anterior visual pathways. What test could best determine this? 2. Optical coherence tomography
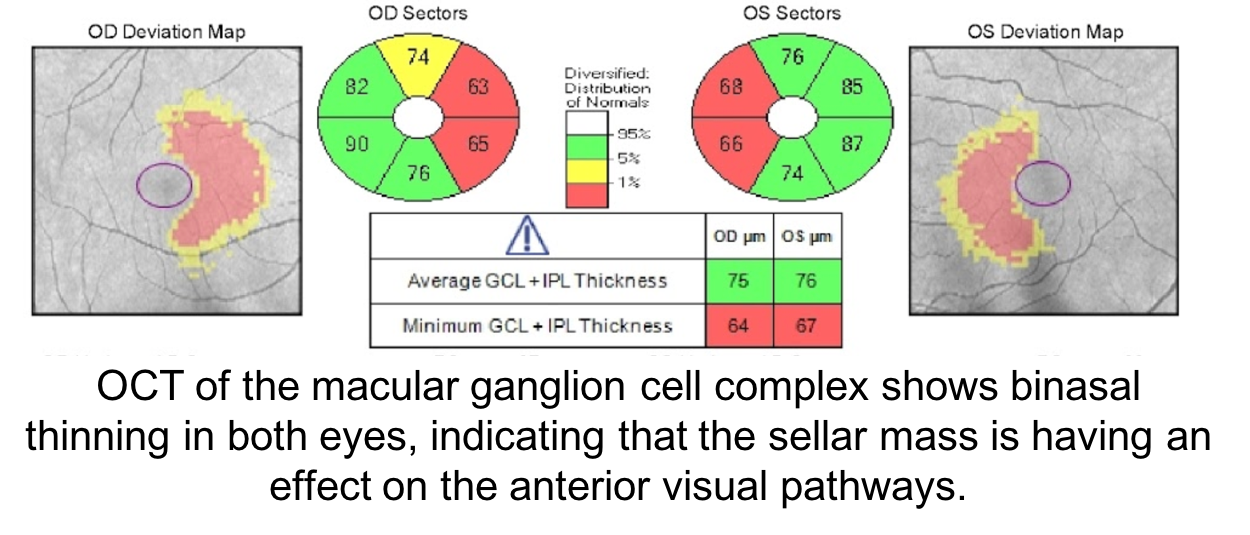
In patients with sellar masses, OCT is not only helpful in prognosticating visual outcome, but is also useful in the diagnosis of compressive chiasmopathies. This is because there may be loss of retinal ganglion cells and retinal nerve fiber layer before visual field defects are seen. Optical coherence tomography is an earlier and more sensitive way of discerning whether there is a compressive chiasmopathy or optic neuropathy. This patient’s OCT of the macular ganglion cell complex showed binasal thinning, indicating that the tumor is having an effect on the visual pathways. Intervening at this stage may prevent vision loss. Optical coherence tomography of the macular ganglion cell complex often shows changes earlier than the RNFL.
12. Which of the following may cause a bitemporal hemianopia without mass effect on the optic chiasm?
- Demyelinating disease
- Aneurysm
- Craniopharyngioma
- Meningioma
12. Which of the following may cause a bitemporal hemianopia without mass effect on the optic chiasm? 1. Demyelinating disease
The most common cause of optic chiasmopathies are pituitary adenomas, suprasellar meningoimas, craniopharyngiomas, and aneurysms of the internal carotid artery, which all have a mass effect on the optic chiasm. In the absence of compression, other less common conditions that affect the optic chiasm include chiasmitis related to multiple sclerosis, sarcoidosis, trauma, radiation, ethambutol toxicity, B12 deficiency, and vasculitis.
13. What neuro-ophthalmic symptoms may be experienced by a patient with a bitemporal hemianopia?
- Monocular diplopia
- Simultagnosia
- Post-fixation blindness and hemifield slide
- Prosopagnosia
13. What neuro-ophthalmic symptoms may be experienced by patients with a bitemporal hemianopia? 3. Post-fixation blindness and hemifield slide
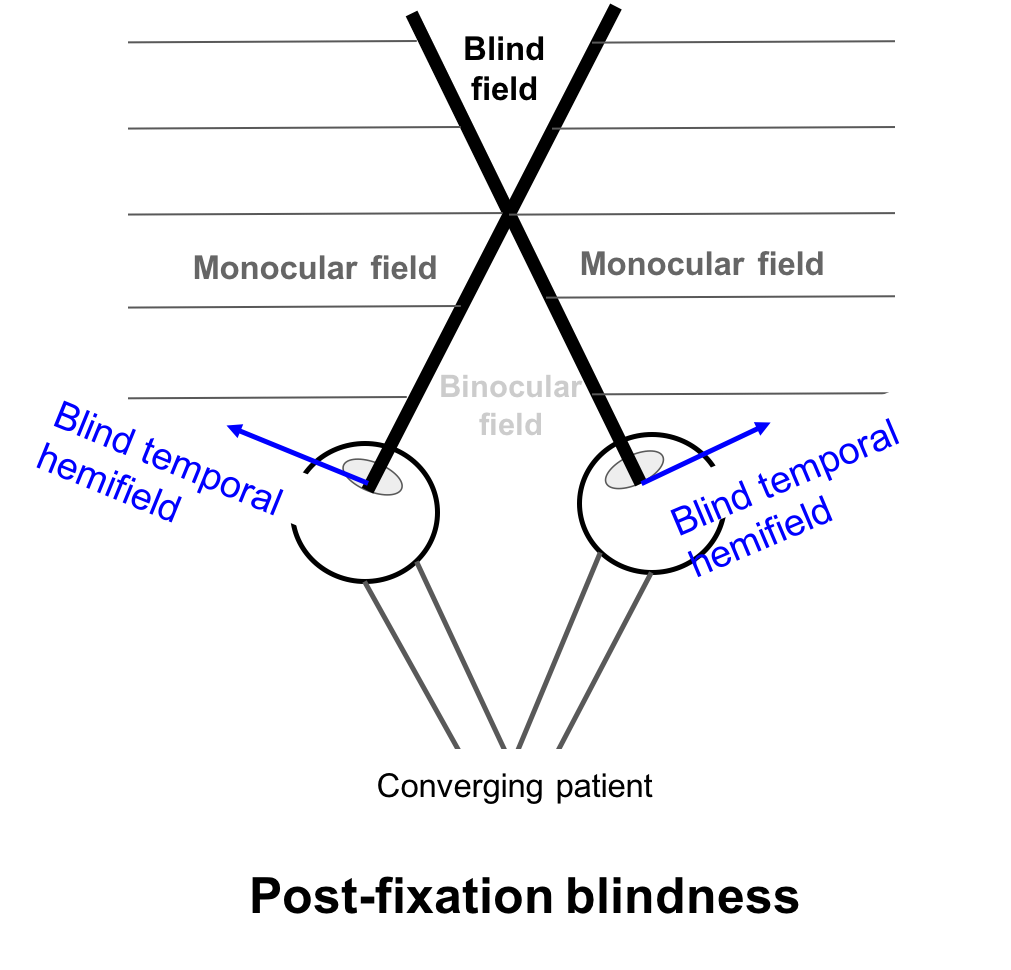
Two visual symptoms that may be experienced by patients with bitemporal hemianopia are post-fixation blindness and hemifield slide.
In post-fixation blindness, a converging patient will have overlap between the two blind temporal hemifields resulting in the disappearance of an object past the fixation point in the blind field. This may cause difficulty in patients performing near work such as threading or using precision tools.
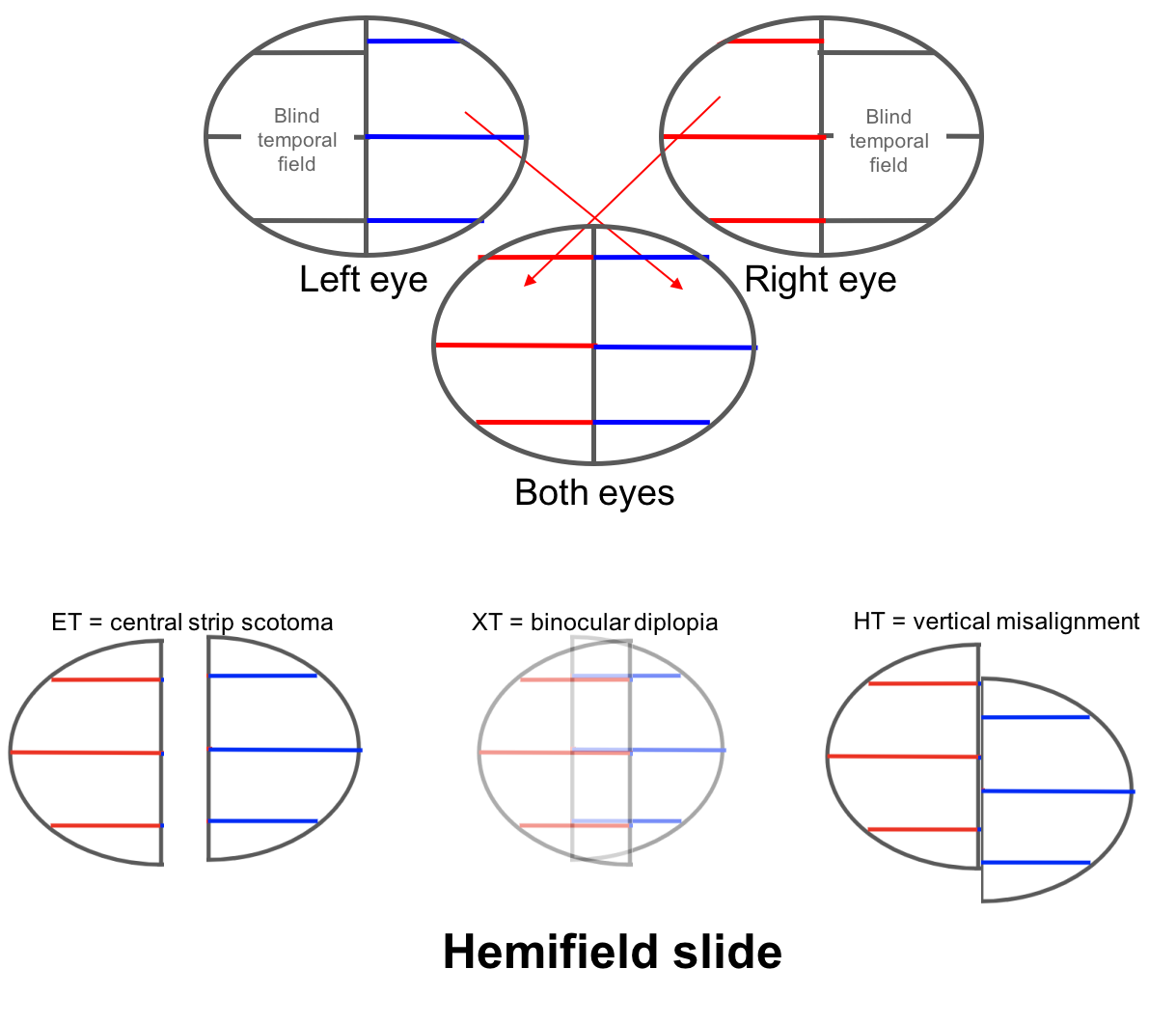
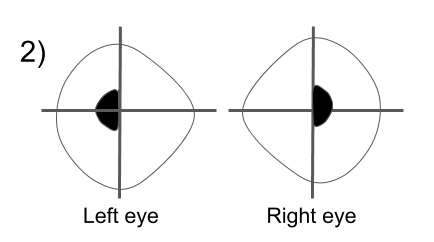
 Hemifield slide is due to the loss of the normal overlap between the two hemifields (nasal field from one eye and temporal field from the other) in a patient with a bitemporal hemianopia. This normal overlap permits fusion of images and helps stabilize ocular alignment in patients with horizontal or vertical phorias. Patients with a preexisting esophoria will become esotropic and have a blind area in the center of their vision, a “central strip scotoma”. Patients with an exophoria will become exotropic and have binocular diplopia and those with a hyperdeviation will complain of separation of images across the vertical meridian as shown below.
Hemifield slide is due to the loss of the normal overlap between the two hemifields (nasal field from one eye and temporal field from the other) in a patient with a bitemporal hemianopia. This normal overlap permits fusion of images and helps stabilize ocular alignment in patients with horizontal or vertical phorias. Patients with a preexisting esophoria will become esotropic and have a blind area in the center of their vision, a “central strip scotoma”. Patients with an exophoria will become exotropic and have binocular diplopia and those with a hyperdeviation will complain of separation of images across the vertical meridian as shown below.
14. Where does this visual field defect localize?
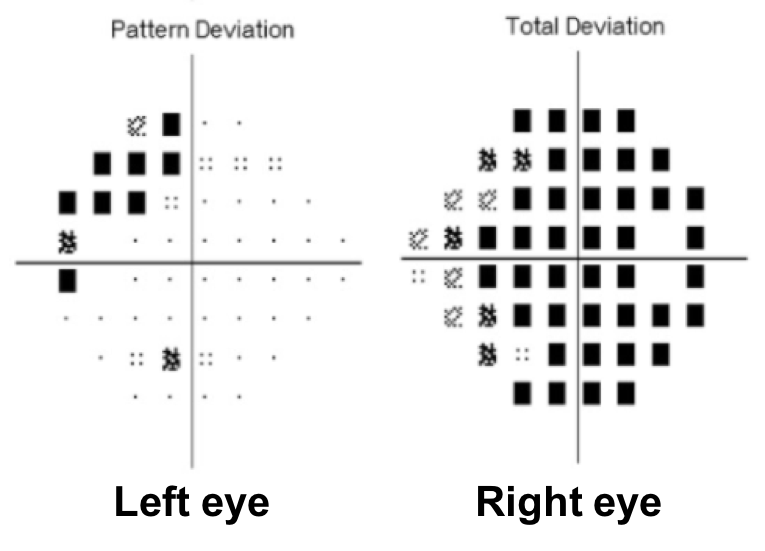
- Right anterior optic chiasm
- Left anterior optic chiasm
- Optic tract
- Posterior optic chiasm
14. Where does this visual field defect localize? 1. Right anterior optic chiasm
This is a junctional scotoma due to a lesion located at the distal portion of the right optic nerve at the angle of the optic chiasm. In this anterior chiasmal syndrome, there is loss of central visual function ipsilateral to the lesion and a contralateral superotemporal or temporal defect. Causes of junctional scotomas are similar to those that cause bitemporal hemianopias. The superotemporal visual field defect in the left eye can be explained by the existence of Wilbrand’s knee, which is a loop of fibers representing this part of the visual field that extends into the contralateral optic nerve before entering the optic tract. However, the existence of Wilbrand’s knee has been has been debated and reported to be an artifact of enucleation.
Clinical Pearl
A complete temporal visual field defect in one eye and a diffuse visual field defect in the fellow eye is referred to as a junctional scotoma.
15. What visual field defect would be expected in a lesion affecting the posterior optic chiasm?
15. What visual field defect would be expected in a lesion affecting the posterior optic chiasm? 2. Bitemportal scotomas (shown below)
Lesions that affect the posterior part of the chiasm cause bitemporal hemianopic scotomas, which are smaller, more central bitemporal defects. Bitemporal scotomas may be confused with bilateral cecocentral scotomas seen in hereditary, toxic or nutritional optic neuropathies. However, cecocentral scotomas are associated with reduced visual acuity and color vision whereas bitemporal scotomas typically have normal visual acuity and color vision.
14. A patient with a pituitary macroadenoma has a subtle right RAPD and the visual field defect shown below. This can be explained by:
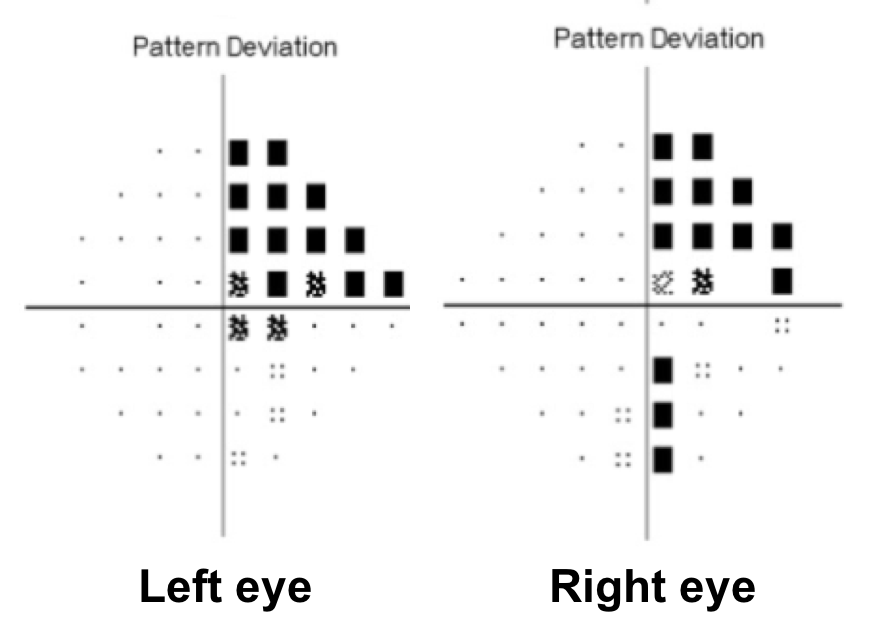
- The presence of a second pituitary adenoma
- A pre-fixed optic chiasm
- A post-fixed optic chiasm
- Extension of the mass anteriorly
14. A patient with a pituitary macroadenoma has a subtle right RAPD and the visual field defect shown below. This can be explained by: 2. A pre-fixed optic chiasm
The optic chiasm can have anatomical variation in its position over the sella. A central chiasm lies directly over the sella and an expanding pituitary adenoma would cause a bitemporal hemianopia. A pre-fixed chiasm lies anterior to the sella (overlying the tuberculum sellae) and an expanding pituitary adenoma may affect the optic tract and cause a homonymous hemianopia. A post-fixed chiasm lies more posterior to the sella (overlying the dorsum sellae) and a pituitary adenoma would affect the pre-chiasmatic optic nerves first. A anatomical study of 60 cadavers found a normally positioned chiasm in 78% of cases, a pre-fixed chiasm in 5% of cases and a post-fixed chiasm in 17% of cases.
Clinical Pearl
A lesion affecting the optic tract will cause the following on the side contralateral to the lesion:
- Homonymous hemianopia
- RAPD
- Bow-tie atrophy of the optic disc
15. Why does an optic tract lesion cause a contralateral RAPD?
- More fibers in the optic tract come from the contralateral eye
- More fibers in the optic tract come from the ipsilateral eye
- The temporal visual field has more pupillary efferent fibers
- There should not be an RAPD
15. Why does an optic tract lesion cause a contralateral RAPD? 2. More fibers in the optic tract come from the contralateral eye
Optic tract lesions often manifest with an RAPD in the eye contralateral to the lesion. This is because there are more fibers in the optic tract from the contralateral eye (classically 53:47) since the temporal visual field is larger than the nasal visual field. This ratio may be even larger for fibers serving the pupillary reflex.
16. Bow-tie atrophy is seen with optic tract lesions. What is bow-tie atrophy?
- Superior and inferior optic atrophy in the eye contralateral to the optic tract lesion
- Nasal and temporal optic atrophy in the eye contralateral to the optic tract lesion
- Nasal and temporal optic atrophy in the eye ipsilateral to the optic tract lesion
- Horizontal optic nerve cupping in the eye contralateral to the optic tract lesion
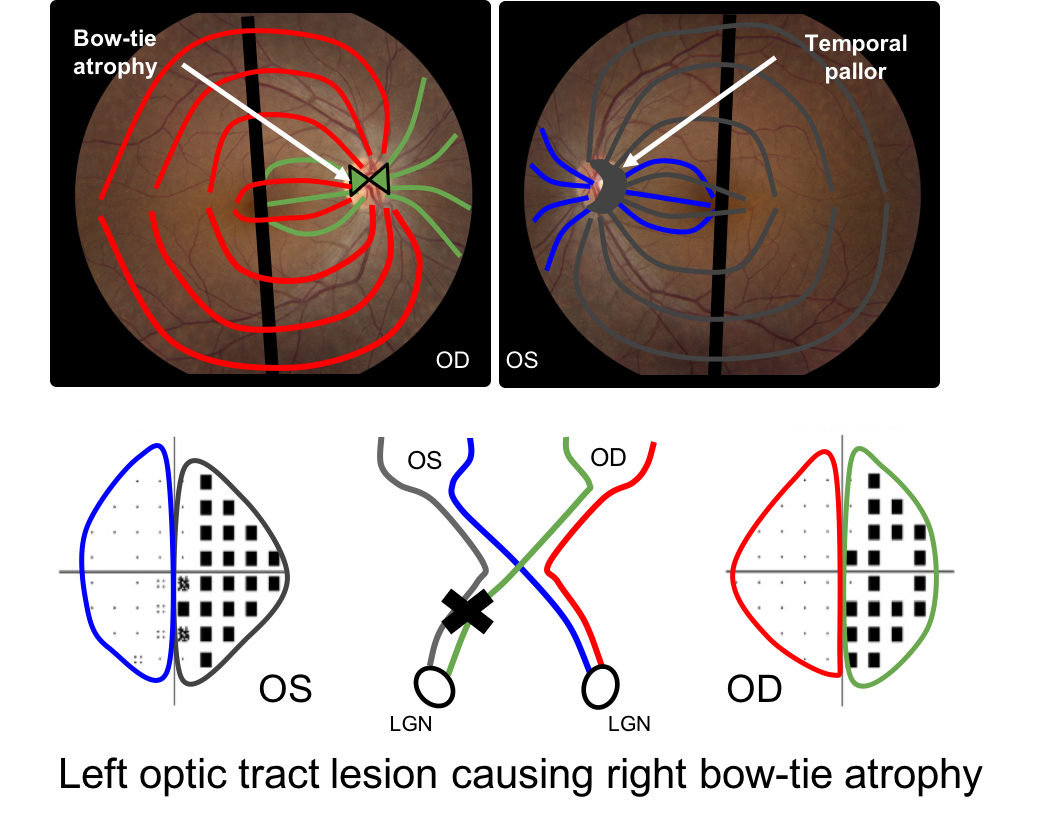
16. Bow-tie atrophy is seen with optic tract lesions. What is bow-tie atrophy? 2. Nasal and temporal optic atrophy in the eye contralateral to the optic tract lesion
There is a single retinal ganglion cell axon that originates in the retina and synapses in the lateral geniculate nucleus. In an optic tract lesion, the retinal ganglion cells in this region die and this results in a specific pattern of optic atrophy seen at the optic nerve head. The eye contralateral to the lesion shows bow-tie atrophy (nasal and temporal atrophy) whereas the eye ispilateral to the lesion usually shows diffuse or temporal pallor. This is illustrated below with a left optic tract lesion. The green fibers in the right eye and the grey fibers in the left eye will atrophy leading to a bow-tie pattern in the right eye and temporal pallor in the left eye.
Case Summary
She had the acute onset of headache following cardiac surgery with vision loss. Examination revealed a bitemporal hemianopia, which was highly concerning for pituitary apoplexy. She became hemodynamically unstable shortly after and was treated with intravenous hydrocortisone for adrenal insufficiency. Magnetic resonance imaging of the brain confirmed the diagnosis of pituitary apoplexy and she underwent transphenoidal resection of the mass. Her visual fields were normal at a 1 month follow-up examination. Her post-operative MRI showed no evidence of residual tumor.
Further reading:
-
Rajasekaran S, Vanderpump M, Baldeweg S, et al. UK guidelines for the management of pituitary apoplexy. Clinical Endocrinology 2001;74:9-20. https://www.ncbi.nlm.nih.gov/pubmed/21044119
-
Johnston PC, Hamrahian AH, Weil RJ, Kennedy L. Pituitary tumor apoplexy. J Clin Neurosci 2015;22(6):939-944. https://cwru.pure.elsevier.com/en/publications/pituitary-tumor-apoplexy-2
-
Hage R, Eshraghi SR, Oyesiku NM, et al. Third, fourth, and sixth cranial nerve palsies in pituitary apoplexy. World Neurosurg 2016;94:447-452. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5064865/
-
Peragallo JH, Bialer OY, Pineles SL, Newman NJ. Hemifield slide phenomenon as a result of heteronymous hemianopia. Neuroophthalmology 2014;38(2):82-87. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5123130/
-
Foroozan R. Chiasmal syndromes. Curr Opin Ophthalmol 2003;14(6):325-31. https://www.ncbi.nlm.nih.gov/pubmed/14615635
-
Kanaan I, Jallu A, Kanaan H. Management strategy for meningioma in pregnancy: a clinical study. Skull Base 2003;13(4):197-203. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1131852/
-
Gulsen S, Hakan A, Unal M, et al. Characterization of the anatomic location of the pituitary stalk and its relationship to the dorsum sellae, tuberculum sellae, and chiasmatic cistern. J Korean Neurosurg Soc 2010;47:169-173. https://www.jkns.or.kr/upload/pdf/0042010034.pdf