8
Case
Age: 34-year-old white man
Reason for referral to ophthalmology: Bilateral vision loss
Past medical history: None
Past ocular history: None
Medications: None
Habits: Smokes ½ pack of cigarettes daily for 10 years, drinks alcohol rarely
HPI: He developed painless vision loss in his right eye 6 months ago and did not seek medical attention. Two weeks ago, he started to notice mildly blurred vision in his left eye. He saw an optometrist who documented a visual acuity of 20/200 OD and 20/25 OS and noted normal appearing optic nerves. He was referred to the emergency room and a CT scan of the head without contrast was performed and was normal. An ophthalmology consultation was requested.
Ophthalmological Examination:
Blood pressure: 120/82, heart rate 75
Visual acuity is 20/200 OD, 20/30 OS
Pupils are equal sizes and reactive to light, there is no RAPD
Color vision is 0/14 correct Ishihara plates in both eyes
Ocular motility and alignment are normal
Slit lamp examination is normal
Neurological examination is normal
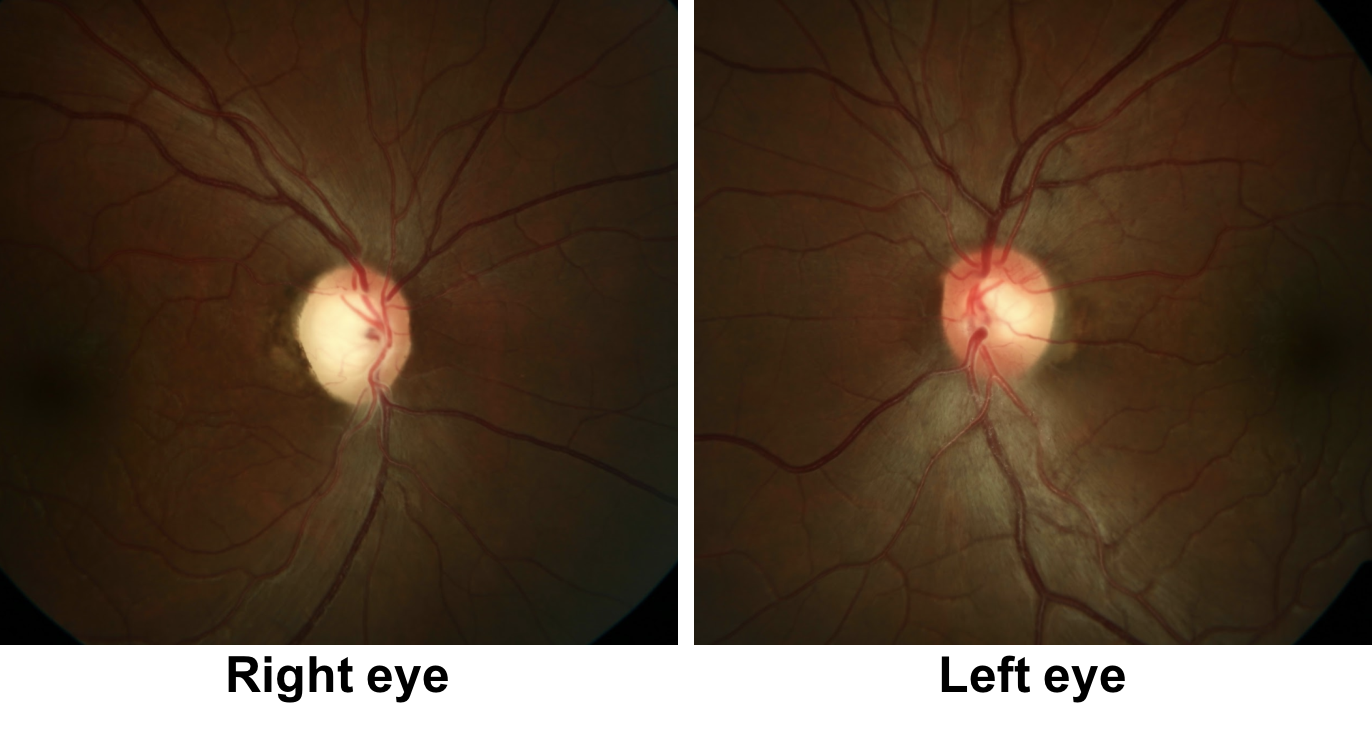
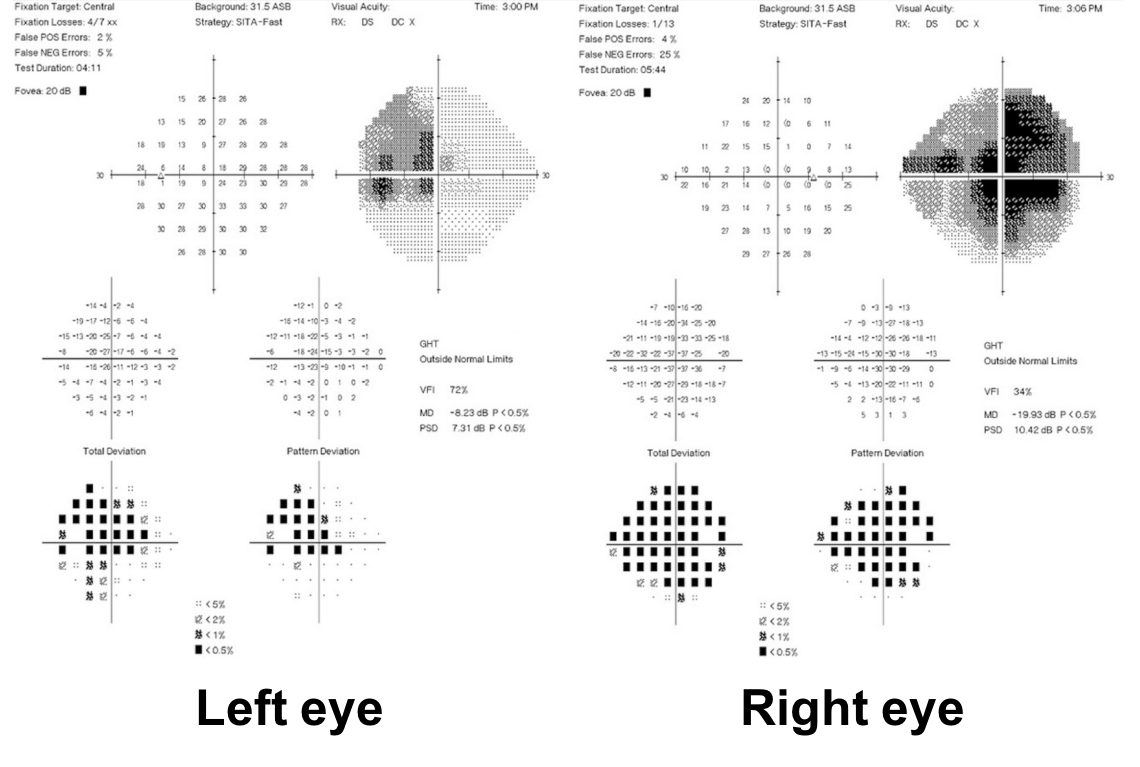
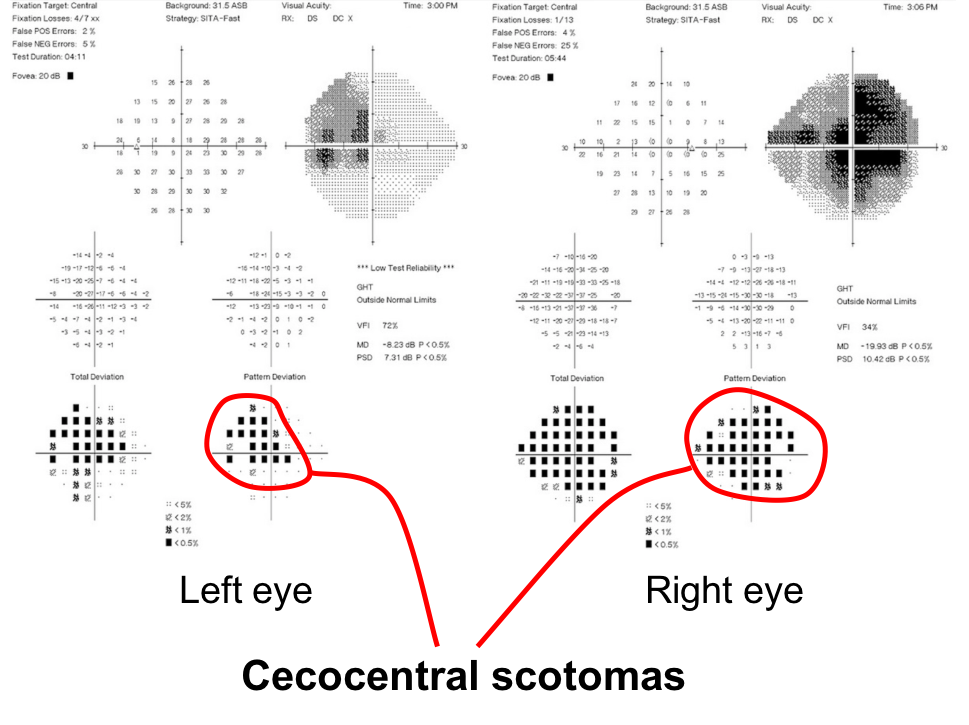
1. What visual field defects does this patient have?
- Bitemporal hemianopia
- Cecocentral scotomas
- Left homonymous hemianopia
- Enlarged blind spots
1. What visual field defects does the patient have? 2. Cecocentral scotomas
A central scotoma that is connected to the blindspot is called a cecocentral scotoma which is present in both eyes of this patient. They are a result of dysfunction of the papillomacular bundle, which is the retinal nerve fiber layer that carries information from the macula to the optic nerve.
Clinical Pearl
The differential diagnosis for bilateral cecocentral scotomas includes:
- Hereditary optic neuropathies (eg. Leber’s Hereditary Optic Neuropathy, Dominant Optic Atrophy)
- Nutritional optic neuropathies (eg. B12, folate, copper deficiency)
- Toxic optic neuropathies (eg. Ethambutol, methanol toxicity)
- Bilateral optic neuritis
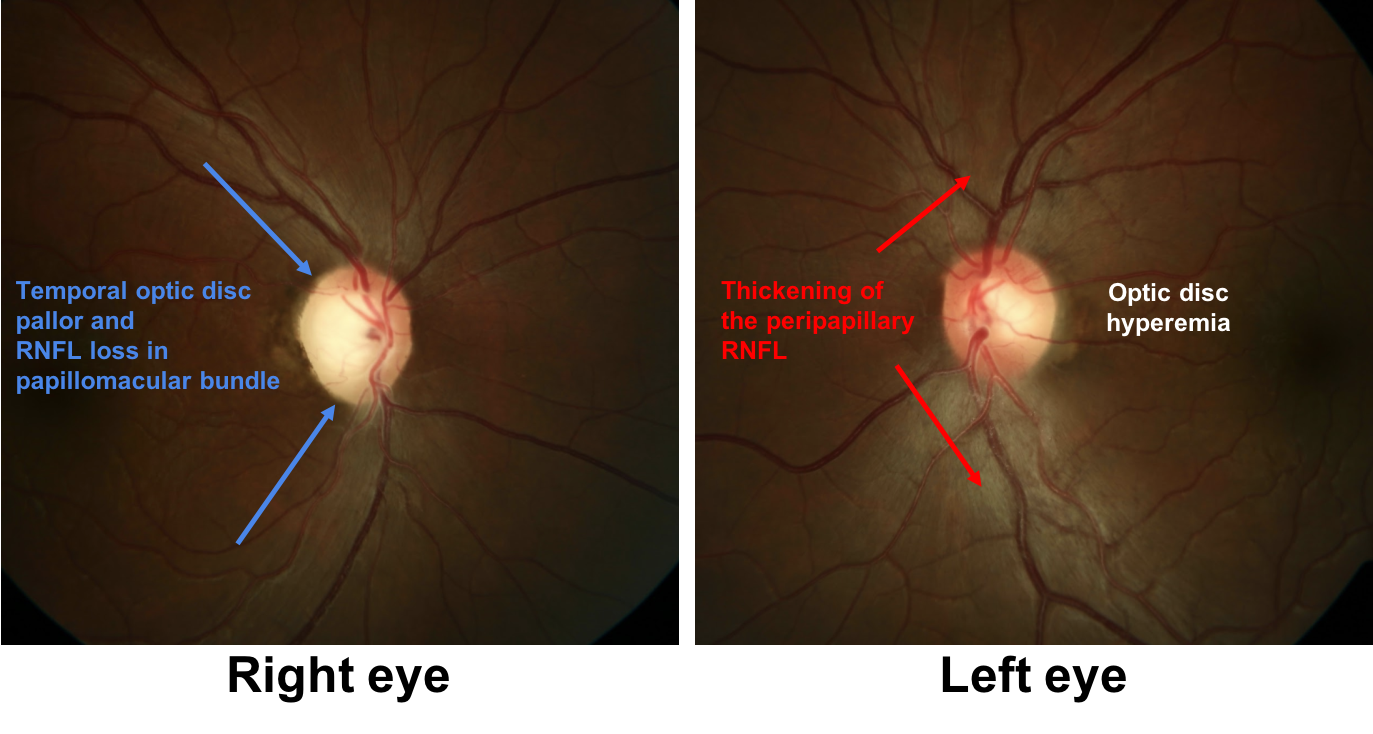
2. The optic disc appearance can be described as:
- Bilateral optic disc edema
- Bilateral optic disc atrophy
- Right temporal pallor and left optic disc hyperemia and thickening of the peripapillary retinal nerve fiber layer
- Left optic disc hemorrhages and right optic disc pallor
2. The optic disc appearance can be described as: 3. Right temporal pallor and left optic disc hyperemia and thickening of the peripapillary retinal nerve fiber layer
There is temporal pallor of the right optic disc, which is consistent with the right eye having lost vision earlier since pallor usually takes at least 6-8 weeks to develop after an insult to the optic nerve, but may be longer in some conditions such as Leber’s Hereditary Optic Neuropathy (LHON). The left optic disc appears hyperemic with thickening of the retinal nerve fiber layer around the optic disc in the left eye.
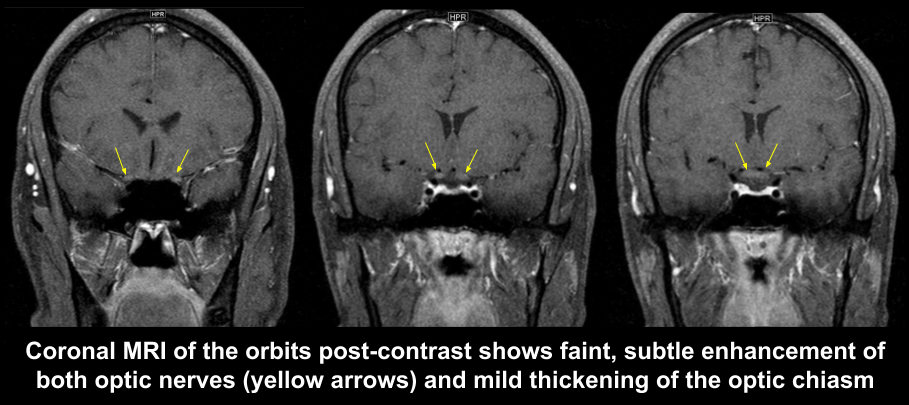
3. What is the most appropriate next step for this patient?
- MRI of the brain and orbits with contrast
- MRA to assess the carotid circulation
- CBC, ESR, and CRP
- None of the above
3. What is the most appropriate next step for this patient? 1. MRI of the brain and orbits with contrast
In this patient with right eye vision loss that has not improved after 6 months and new onset vision loss in the left eye, it is important to evaluate the patient for compressive and inflammatory optic neuropathies. This is best done with an MRI of the brain and orbits with contrast. Representative images from the MRI of the orbits is shown below and shows subtle enhancement and thickening of optic nerves and chiasm. There is no compressive lesion of the anterior visual pathways.
The patient was admitted to hospital and the following investigations were obtained:
- CSF: no nucleated cells, normal protein and glucose
- Angiotensin converting enzyme: normal
- Chest x-ray: normal
- Syphilis testing: normal
He was treated with intravenous methylprednisolone 1 gram daily, but he continued to lose vision.
- AQP4 and MOG antibodies: negative
4. What is the next most appropriate test to obtain in this patient?
- Genetic testing
- CT scan of the orbits
- Repeat lumbar puncture
- Magnetic Resonance Venography (MRV)
4. What is the next most appropriate test to obtain in this patient? 1. Genetic testing
This is a young man who had bilateral sequential vision loss with subtle enhancement of the optic nerves on MRI and negative NMO and MOG antibodies. The most likely diagnosis is Leber’s Hereditary Optic Neuropathy (LHON) and genetic testing for the 3 primary mutations in mitochondrial DNA should be obtained: 11778, 14484, 3460. The most common mutation is 11778 (69% of cases), 14484 (about 14% of cases), and 3460 (about 13% of cases).
The patient tested positive for the 11778 mutation and was diagnosed with LHON
MRI of the orbits demonstrated chiasmal enlargement and mild enlargement and enhancement of the proximal, intracranial optic nerves, which may be seen in LHON.
Clinical Pearl
The following 3 mutations account for 90% of all LHON cases:
-
-
- 11778 (ND4 gene)
- 14484 (ND6 gene)
- 3460 (ND1 gene)
-
5. What would be expected if the patient had an intravenous fluorescein angiogram?
- Leakage from the left optic disc only
- Leakage from both optic discs
- No leakage from either optic disc
- Enlargement of the foveal avascular zone
5. What would be expected if the patient had an intravenous fluorescein angiogram? 3. No leakage from either optic disc
The classic description of the optic disc in LHON includes absence of disc leakage with fluorescein angiography. The classic triad of ocular fundus changes is listed below (Clinical Pearl). Although these fundus findings are helpful when present, in up to 50% of LHON cases, the optic disc appears normal.
Clinical Pearl
The classic triad of ocular fundus changes seen in LHON:
- circumpapillary telangiectactic microangiopathy
- elevation of the retinal nerve fiber layer around the optic disc
- absence of disc leakage on fluorescein angiography
6. What treatment can be considered in this patient?
- Rituximab
- Idebenone
- Tetracycline
- Vitamin A
6. What treatment can be considered in this patient? 2. Idebenone
General therapies that have been suggested for the treatment of mitochondrial disease such as LHON include vitamins and cofactors (coenzyme Q10, folic acid, thiamine, riboflavin, L-arginine), electron acceptors (vitamin C), and free radical scavengers (coenzyme Q10, idebenone). There is very limited evidence for the effectiveness of these therapies. Coenzyme Q10 is a mitochondrial cofactor that shuttles electrons from complex I and II to complex III, but exogenous coenzyme Q10 treatment is limited in effectiveness since it has poor ability to cross lipid membranes to the mitochondria.
Idebenone is a coenzyme Q10 derivative that was reported to have higher delivery to mitochondria and higher efficiency in crossing the blood-brain-barrier. A 24-week international, double-blind, randomized placebo-controlled trial of 85 patients with LHON with vision loss up to 5 years showed that idebenone 900 mg daily did not show statistical significance in the primary end point (best recovery of visual acuity) or various secondary end points. However, post hoc subgroup analysis showed that patients with a discordant visual acuity (difference of logMAR >0.2) at baseline and treated with idebenone showed statistically significant improvement in secondary endpoints compared to placebo groups. Since this treatment was tolerated well and safe, it is reasonable to recommend idebenone for this patient. It is highly recommended that he quit smoking and avoid binge alcohol drinking, which have been reported as precipitants of vision loss in LHON.
7. What are good prognostic factors for visual recovery in patients with LHON?
- 11778 mutation and older age of onset (older than 40 years of age)
- 14484 mutation and younger age of onset (less than 20 years of age)
- 14484 mutation and older age of onset (older than 30 years of age)
- 11778 mutation and younger age of onset (less than 20 years of age)
7. What are good prognostic factors for visual recovery in patients with LHON? 2. 14484 mutation and younger age of onset (less than 20 years of age)
Spontaneous visual recovery is heavily influenced by the underlying LHON mutation. The 14484 mutation has a 37-71% chance of some degree of visual improvement whereas the 11778 mutation has only a 4% chance. Age of onset less than 20 years of age has also been reported as a positive prognostic feature. This patient was positive for the 11778 mutation and has a worse visual prognosis.
8. What is the median time between vision loss in both eyes in patients with LHON?
- 4-5 days
- 1-2 weeks
- 6-8 weeks
- 4-6 months
8. What is the median time between vision loss in both eyes in patients with LHON? 3. 6-8 weeks
More than 97% of patients with LHON develop vision loss in the second eye within 1 year. The median delay of onset between the two eyes is 6-8 weeks. Bilateral simultaneous loss of vision has been reported in up to 50% of cases, but this may also include patients with unrecognized vision loss in the first eye.
9. What percentage of patients with LHON are men?
- 100%
- 80-90%
- 50-60%
- 20-30%
9. What percentage of patients with LHON are men? 2. 80-90%
Most pedigrees show a male predominance of 80-90%. Gender bias in LHON remains an enigma, but may be related to the presence of estrogen and its ability to increase mitochondrial biogenesis to compensate for the defective gene.
10. This patient (a 34-year-old man) is planning on having children, what is his chance of passing the LHON gene to his children?
- 0%
- 25%
- 50%
- 100%
10. This patient (a 34 year old man) is planning on having children, what is his chance of passing the LHON gene to his children? 1. 0%
LHON is a maternally inherited disease. The mitochondrial genome is transferred from a mother to all of her children and there is no paternal contribution. This patient has a 40-year-old brother that did not have any vision loss, emphasizing the point that a LHON mutation is necessary but not sufficient to cause vision loss. This may be accounted for by heteroplasmy (coexistence of both wild-type and mutant mitochondrial DNA in the same mitochondrion), additional nuclear genetic factors, mitochondrial genetic factors, and environmental factors.
Clinical Pearl
LHON is passed through maternal family members and is not transmitted from a father to his children. A typical LHON pedigree is shown below. Notice how only the men (squares) are affected and men do not transmit the disease to their offspring.
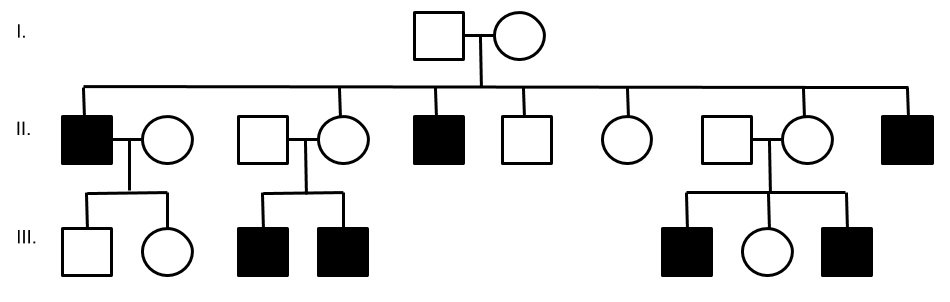
11. What is the age of the youngest and oldest individuals reported to have vision loss from LHON?
- 2 and 87 years old
- 15 and 35 years old
- 10 and 53 years old
- 15 and 77 years old
11. What is the age of the youngest and oldest individuals reported to have vision loss from LHON? 1. 2 and 87 years old
The onset of symptoms usually occurs between 15 and 35 years of age, but the youngest and older ages reported are 2 and 87 years old, respectively. LHON should still be considered in the differential diagnosis for vision loss in older individuals.
12. What other test should be considered for this patient with LHON?
- EKG
- CT chest
- Repeat lumbar puncture
- Nerve conduction studies
12. What other test should be considered for this patient with LHON? 1. EKG
In some pedigrees of LHON, cardiac conduction defects may occur. These include long QT syndromes and Wolff-Parkinson-White syndrome. An EKG should be performed in LHON patients to screen for these abnormalities. A family history of sudden cardiac arrest may give a clue to the diagnosis of LHON or other mitochondrial diseases.
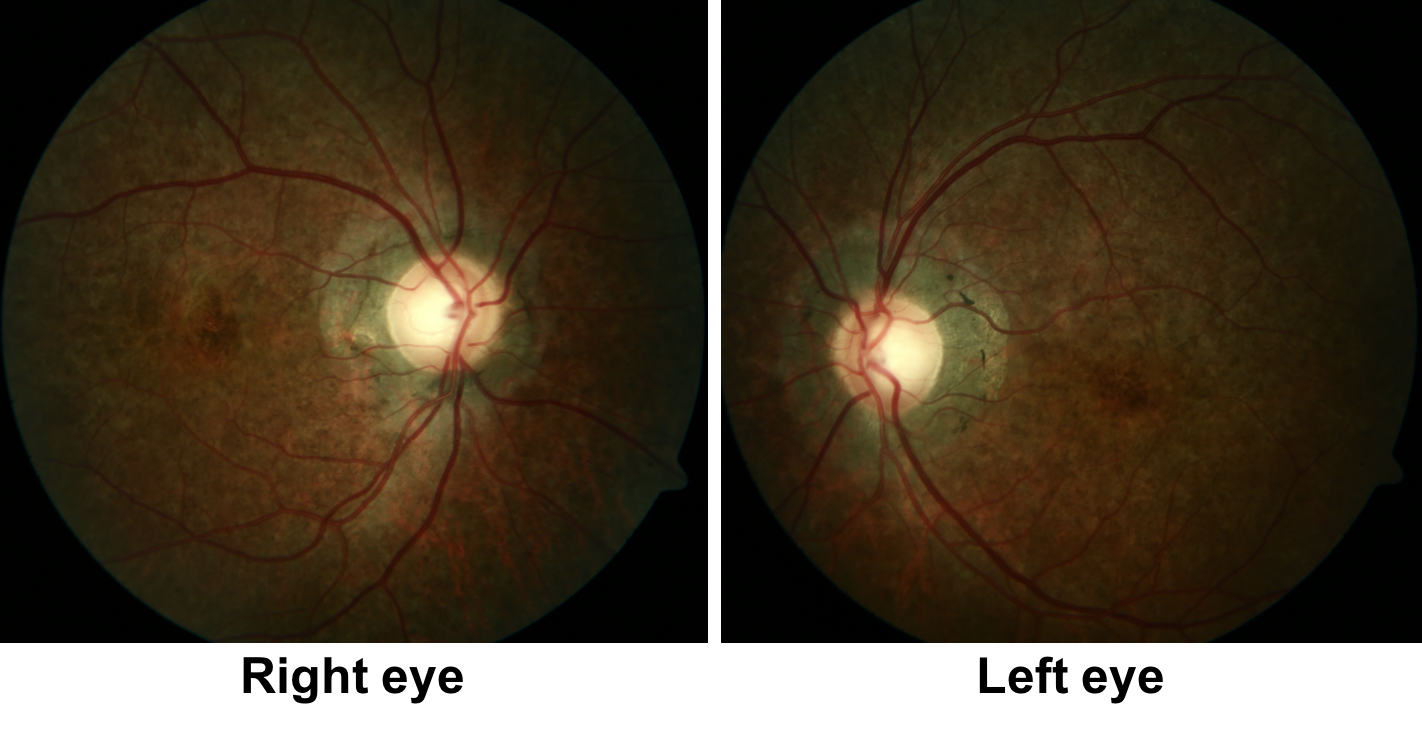
13. A 22-year-old man has a history of heart block and a pacemaker was inserted 4 years ago. He developed bilateral, symmetrical ptosis and ophthalmoplegia over several years. Fundus photos are shown below. What is the most likely diagnosis?
- LHON
- Myasthenia gravis
- Sarcoidosis
- Kearns-Sayre Syndrome
13. A 28-year-old man has a history of heart block and a pacemaker was inserted 8 years ago. He developed bilateral, symmetrical ptosis and ophthalmoplegia over several years. Fundus photos are shown below. What is the most likely diagnosis? 4. Kearns-Sayre Syndrome
This young patient has cardiac conduction defects, insiduous painless progressive bilateral ptosis and ophthalmoparesis, and bilateral pigmentary retinopathy. This is characteristic of Kearns-Sayre Syndrome, a mitochondrial disorder, which refers to the combination of chronic progressive external ophthalmoplegia (CPEO) and pigmentary retinopathy. The diagnosis can be confirmed with a muscle biopsy, which typically shows ragged red fibers on Gomori trichrome stain, which is a characteristic sign of mitochondrial disease.
14. Dominant Optic Atrophy (DOA) is caused by a mutation in:
- Mitchondrial DNA
- Nuclear DNA
- X-chromosome
- None of the above
14. Dominant Optic Atrophy (DOA) is caused by a mutation in: 2. Nuclear DNA
The majority of cases of DOA are caused by mutations in the OPA1 gene, which is located on chromosome 3. The OPA1 gene is located on nuclear DNA but is expressed in the mitochondria and encodes a dynamin-related GTPase that is located on the inner membrane of mitochondria cristae. Unlike LHON, over 200 mutations in the OPA1 gene have been identified.
15. How do patients with Dominant Optic Atrophy (DOA) typically present?
- Sudden loss of vision in one eye followed by sudden loss in the other
- Sudden loss of vision in both eyes simultaneously
- Slowly progressive, bilateral vision loss
- Slowly progressive vision loss followed by acute exacerbations
15. How do patients with Dominant Optic Atrophy (DOA) typically present? 3. Slowly progressive, bilateral vision loss
The typical presentation of a patient with DOA is slowly progressive, bilateral, symmetric vision loss, which usually begins in the first two decades of life. Since the vision loss is slowly progressive, many patients are unaware of the vision loss initially and cannot pinpoint a precise date of onset. Vision loss in DOA is much milder than that seen in LHON with a mean visual acuity of 20/80 to 20/120 with more than 80% of patients being 20/200 or better.
16. What are the clinical manifestations of Wolfram syndrome?
- Optic atrophy
- Diabetes mellitus
- Diabetes insipidus
- Deafness
- All of the above
16. What are the clinical manifestations of Wolfram syndrome? 5. All of the above
Wolfram syndrome is a rare neurodegenerative disorder characterized by juvenile onset type 2 diabetes, progressive bilateral optic neuropathy, diabetes insipidus, and sensorineural hearing loss. It is sometimes referred to as DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness). The first manifestation of the disease is usually diabetes mellitus in the first or second decade of life followed by bilateral optic neuropathies. The optic neuropathy resembles that of dominant optic atrophy in that there is bilateral insidious loss of vision (usually worse than 20/200), loss of central and peripheral visual fields and excavation of the optic disc. It is usually inherited in an autosomal recessive fashion and one causative gene, WFS1, has been found on chromosome 4p16.1.
Case Summary
He had loss of vision in the right eye 6 months ago and a recent loss of vision in the left eye. His right eye had optic disc pallor consistent with the previous vision loss and there was hyperemia and thickening of the peripapillary retinal nerve fiber layer in the left eye. Magnetic resonance imaging of the brain and orbits with contrast showed subtle enhancement of the optic nerves and genetic testing was positive for the 11778 mutation causing Leber’s Hereditary Optic Neuropathy (LHON). Treatment with Idebenone 900 mg daily was initiated the importance of avoiding smoking and alcohol binge drinking was discussed. Since he has a 40-year-old brother, it was also recommended that he avoid smoking and binge drinking. A 6 month follow-up appointment was arranged. A low vision consultation was requested and the local state laws for driving were reviewed and he did not meet the driving requirements. At his 6 month follow-up appointment, his visual acuity was 20/400 in both eyes and both his optic nerves appeared pale. He was using low vision aids to help with his activities of daily living.

Further reading:
-
Fraser JA, Biousse V, Newman NJ. The neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol 2010;55(4):229-334. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2989385/
-
Klopstock T, Wai-Man Y, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber’s Hereditary Optic Neuropathy. Brain 2011;134(Pt 9):2677-86. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3170530/
-
Kogachi K, Te-Zakarian A, Tian J, Karanjia R, Sadun A. The elusive pathophysiology of Leber’s Hereditary Optic Neuropathy. Vis Pan-Am 2016;15(4):102-105. http://journals.sfu.ca/paao/index.php/journal/article/viewFile/352/pdf
-
Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 2004;23(1):53-89. https://www.ncbi.nlm.nih.gov/pubmed/14766317
-
Phillips PH, Vaphiades M, Glasier CM et al. Chiasmal enlargement and optic nerve enhancement on magnetic resonance imaging in Leber Hereditary Optic Neuropathy. Arch Ophthalmol 2003;121(4):577-579. https://jamanetwork.com/journals/jamaophthalmology/fullarticle/415217