Introduction to Physics
Last Update: 08/18/2021
Physical Quantities and Units
We define a physical quantity either by specifying how it is measured or by stating how it is calculated from other measurements. For example, we define distance and time by specifying methods for measuring them, whereas we define average speed by stating that it is calculated as distance traveled divided by time of travel.
Measurements of physical quantities are expressed in terms of units, which are standardized values. For example, the length of a race, which is a physical quantity, can be expressed in units of meters (for sprinters) or kilometers (for distance runners). Without standardized units, it would be extremely difficult for scientists to express and compare measured values in a meaningful way.
There are two major systems of units used in the world: SI units (also known as the metric system) and English units (also known as the customary or imperial system). English units were historically used in nations once ruled by the British Empire and are still widely used in the United States. Virtually every other country in the world now uses SI units as the standard; the metric system is also the standard system agreed upon by scientists and mathematicians. The acronym “SI” is derived from the French Système International.
SI Units: Fundamental and Derived Units
The table below gives the fundamental SI units that are used throughout this textbook.
| Length | Mass | Time | Electric Current |
|---|---|---|---|
| meter (m) | kilogram (kg) | second (s) | ampere (A) |
In this textbook, the fundamental physical quantities are taken to be the length, mass, time, and electric current. All other physical quantities, such as force and electric charge, can be expressed as algebraic combinations of length, mass, time, and current (for example, speed is length divided by time); these units are called derived units.
Metric Prefixes
SI units are part of the metric system. The metric system is convenient for scientific and engineering calculations because the units are categorized by factors of 10. The table below gives metric prefixes and symbols used to denote various factors of 10.
Metric systems have the advantage that conversions of units involve only powers of 10. There are 100 centimeters in a meter, 1000 meters in a kilometer, and so on. In nonmetric systems, such as the system of U.S. customary units, the relationships are not as simple—there are 12 inches in a foot, 5280 feet in a mile, and so on. Another advantage of the metric system is that the same unit can be used over extremely large ranges of values simply by using an appropriate metric prefix. For example, distances in meters are suitable in construction, while distances in kilometers are appropriate for air travel, and the tiny measure of nanometers are convenient in optical design. With the metric system, there is no need to invent new units for particular applications.
The term order of magnitude refers to the scale of a value expressed in the metric system. Each power of 10 in the metric system represents a different order of magnitude. For example, 101, 102, 103, and so forth are all different orders of magnitude. All quantities that can be expressed as a product of a specific power of 10 are said to be of the same order of magnitude. For example, the number 800 can be written as 8×102, and the number 450 can be written as 4.5×102 Thus, the numbers 800 and 450 are of the same order of magnitude: 102 Order of magnitude can be thought of as a ballpark estimate for the scale of a value. The diameter of an atom is on the order of 10-9m while the diameter of the Sun is on the order of 109m.
| Prefix | Symbol | Value1 | Example (some are approximate) | |||
|---|---|---|---|---|---|---|
| exa | E | 1018 | exameter | Em | 1018 m | the distance light travels in a century |
| peta | P | 1015 | petasecond | Ps | 1015 s | 30 million years |
| tera | T | 1012 | terawatt | TW | 1012 w | powerful laser output |
| giga | G | 109 | gigahertz | GHz | 109 Hz | a microwave frequency |
| mega | M | 106 | megacurie | MCi | 106 Ci | high radioactivity |
| kilo | k | 103 | kilometer | km | 103 m | about 6/10 mile |
| hecto | h | 102 | hectoliter | hL | 102 L | 26 gallons |
| deka | da | 101 | dekagram | dag | 101 g | teaspoon of butter |
| — | — | 100(=1) | ||||
| deci | d | 10-1 | deciliter | dL | 10-1 L | less than half a soda |
| centi | c | 10-2 | centimeter | cm | 10-2 m | fingertip thickness |
| milli | m | 10-3 | millimeter | mm | 10-3 m | flea at its shoulders |
| micro | µ | 10-6 | micrometer | µm | 10-6 m | detail in microscope |
| nano | n | 10-9 | nanogram | ng | 10-9 g | small speck of dust |
| pico | p | 10-12 | picofarad | pF | 10-12 F | small capacitor in radio |
| femto | f | 10-15 | femtometer | fm | 10-15 m | size of a proton |
| atto | a | 10-18 | attosecond | as | 10-18 s | time light crosses an atom |
Unit Conversion and Dimensional Analysis
Let us consider a simple example of how to convert units. Let us say that we want to convert 80 meters (m) to kilometers (km).
The first thing to do is to list the units that you have and the units that you want to convert to. In this case, we have units in meters and we want to convert them to kilometers.
Next, we need to determine a conversion factor relating meters to kilometers. A conversion factor is a ratio expressing how many of one unit is equal to another unit. For example, there are 12 inches in 1 foot, 100 centimeters in 1 meter, 60 seconds in 1 minute, and so on. In this case, we know that there are 1,000 meters in 1 kilometer.
Now we can set up our unit conversion. We will write the units that we have and then multiply them by the conversion factor so that the units cancel out, as shown:
![]()
Example1.1 – Unit Conversion – A Short Drive Home
Suppose that you drive the 10.0 km from your university to home in 20.0 min. Calculate your average speed (a) in kilometers per hour (km/h) and (b) in meters per second (m/s). (Note: Average speed is the distance traveled divided by time of travel.)
Strategy
First, we calculate the average speed using the given units. Then we can get the average speed into the desired units by picking the correct conversion factor and multiplying by it. The correct conversion factor is the one that cancels the unwanted unit and leaves the desired unit in its place.
Solution for (a)
(1) Calculate average speed. Average speed is the distance traveled divided by time of travel. In equation form,
![]()
(2) Substitute the given values for distance and time.
![]()
(3) Convert km/min to km/h: multiply by the conversion factor that will cancel minutes and leave hours. That conversion factor is 60 min/hour. Thus,
![]()
Discussion for (a)
To check your answer, consider the following:
(1) Be sure that you have properly cancelled the units in the unit conversion. If you have written the unit conversion factor upside down, the units will not cancel properly in the equation. If you accidentally get the ratio upside down, then the units will not cancel; rather, they will give you the wrong units as follows:
![]()
which are obviously not the desired units of km/h.
(2) Check that the units of the final answer are the desired units. The problem asked us to solve for average speed in units of km/h and we have indeed obtained these units.
(3) Check the significant figures. Because each of the values given in the problem has three significant figures, the answer should also have three significant figures. The answer 30.0 km/hr does indeed have three significant figures, so this is appropriate. Note that the significant figures in the conversion factor are not relevant because an hour is defined to be 60 minutes, so the precision of the conversion factor is perfect.
(4) Next, check whether the answer is reasonable. Let us consider some information from the problem—if you travel 10 km in a third of an hour (20 min), you would travel three times that far in an hour. The answer does seem reasonable.
Solution for (b)
There are several ways to convert the average speed into meters per second.
(1) Start with the answer to (a) and convert km/h to m/s. Two conversion factors are needed—one to convert hours to seconds, and another to convert kilometers to meters.
(2) Multiplying by these yields
![]()
![]()
Discussion for (b)
If we had started with 0.500 km/min, we would have needed different conversion factors, but the answer would have been the same: 8.33 m/s.
Example 1.2 – Dosage Calculation
An MD orders 300 mg of Ibuprofen to be taken by a 6 kg infant every 4 hours. The label
shows 75 – 150 mg/kg per day max. Is the physician’s order within normal range?
First let’s calculate the max dosage for this infant:
![]()
Now let’s calculate the ordered dose.
![]()
The dosage is not within the normal range.
Accuracy, Precision, and Uncertainty of a Measurement
Science is based on observation and experiment—that is, on measurements. Accuracy is how close a measurement is to the correct value for that measurement. For example, let us say that you are measuring the length of standard computer paper. The packaging in which you purchased the paper states that it is 11.0 inches long. You measure the length of the paper three times and obtain the following measurements: 11.1 in., 11.2 in., and 10.9 in. These measurements are quite accurate because they are very close to the correct value of 11.0 inches. In contrast, if you had obtained a measurement of 12 inches, your measurement would not be very accurate.
The precision of a measurement refers to how close the agreement is between repeated measurements (which are repeated under the same conditions). Consider the example of the paper measurements. The precision of the measurements refers to the spread of the measured values. One way to analyze the precision of the measurements would be to determine the range, or difference, between the lowest and the highest measured values. In that case, the lowest value was 10.9 in. and the highest value was 11.2 in. Thus, the measured values deviated from each other by at most 0.3 in. These measurements were relatively precise because they did not vary too much in value. However, if the measured values had been 10.9, 11.1, and 11.9, then the measurements would not be very precise because there would be significant variation from one measurement to another.
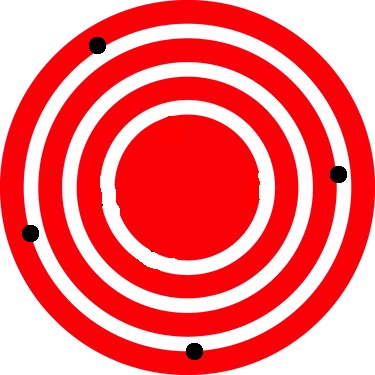
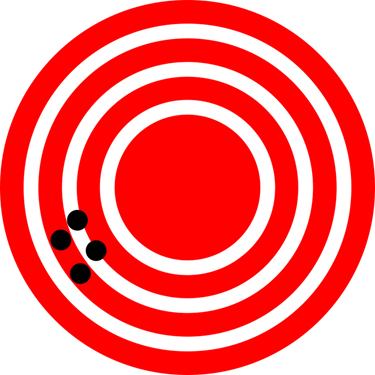
The measurements in the paper example are both accurate and precise, but in some cases, measurements are accurate but not precise, or they are precise but not accurate. Let us consider an example of a GPS that is attempting to locate the position of a restaurant in a city. Think of the restaurant location as existing at the center of a bulls-eye target, and think of each GPS attempt to locate the restaurant as a black dot. In figure 1.1, you can see that the GPS measurements are spread out far apart from each other, but they are all relatively close to the actual location of the restaurant at the center of the target. This indicates a low precision, but high accuracy. However, in figure 1.2, the GPS measurements are concentrated quite closely to one another, but they are far away from the target location. This indicates a high precision, low accuracy measuring system.
Figure 1.1
|
Low Precision-High Accuracy
|
High Precision – Low Accuracy | |
 |
 |
The factors contributing to uncertainty in a measurement include:
- Limitations of the measuring device,
- The skill of the person making the measurement,
- Irregularities in the object being measured,
- Any other factors that affect the outcome (highly dependent on the situation).
In our example, such factors contributing to the uncertainty could be the following: the smallest division on the ruler is 0.1 in., the person using the ruler has bad eyesight, or one side of the paper is slightly longer than the other. At any rate, the uncertainty in measurement must be based on careful consideration of all the factors that might contribute and their possible effects.
Percent Uncertainty
One method of expressing uncertainty is as a percent of the measured value. If a measurement A is expressed with uncertainty, ∂A, the percent uncertainty (%unc) is defined to be
![]()
Example 1.3 – Calculating Percent Uncertainty: A Bag of Apples
A grocery store sells 5lb bags of apples. You purchase four bags over the course of a month and weigh the apples each time. You obtain the following measurements:
- Week 1 weight: 4.8 lb
- Week 2 weight: 5.3 lb
- Week 3 weight: 4.9 lb
- Week 4 weight: 5.4 lb
You determine that the weight of the 5-lb bag has an uncertainty of ±0.4lb. What is the percent uncertainty of the bag’s weight?
Strategy
First, observe that the expected value of the bag’s weight, A, is 5 lb. The uncertainty in this value ∂A is 0.4 lb. We can use the following equation to determine the percent uncertainty of the weight:
Solution
Plug the known values into the equation:
Discussion
We can conclude that the weight of the apple bag is 5lb±8%. Consider how this percent uncertainty would change if the bag of apples were half as heavy, but the uncertainty in the weight remained the same. Hint for future calculations: when calculating percent uncertainty, always remember that you must multiply the fraction by 100%. If you do not do this, you will have a decimal quantity, not a percent value.
Uncertainties in Calculations
There is uncertainty in anything calculated from measured quantities. For example, the area of a floor calculated from measurements of its length and width has uncertainty because the length and width have uncertainties. Before we discuss how to include uncertainties in calculations, we need to understand the difference between absolute uncertainty and percent (or relative) uncertainty.
Suppose the length and width of a rectangular floor are measured to be 4.00m±0.02m and 2.25m±0.05m. The values 0.02m and 0.05m are absolute uncertainties. Absolute uncertainty is expressed in the same unit as the measurement. Also, the precision of the absolute uncertainty must match the precision of the measurement. In other words, the measurement and its absolute uncertainty must have the same number of digits past the decimal point.
We can also find the % uncertainty for each measurement as following.
![]()
![]()
Therefore, there are two ways to express the uncertainty in a measurement – in absolute form or in % form.
![]()
![]()
The following rules can be used to determine the uncertainty in calculations as long as the measurements going into the calculation have small uncertainties (a few percent or less).
Addition or Subtraction
The rule is: When adding or subtracting measured quantities with uncertainties, add the absolute uncertainties.
If we wanted to determine the length of the fencing needed to cover two adjacent sides of the floor mentioned above, we would have to add the length and the width.
![]()
![]()
![]()
If we wanted to find the difference between the length and the width, we would have to do a subtraction.
![]()
![]()
![]()
Notice that in both cases we add the absolute uncertainties.
Multiplication and division
The rule is: When multiplying or dividing measured quantities with uncertainties, add the % uncertainties.
To find the area of this floor we have to multiply the width and the length.
![]()
![]()
![]()
To express the uncertainty int he Area in absolute form, we need to figure out what 2.7% of 9.00m is.
![]()
So the result can also be written as
![]()
Notice that we would still add the % uncertainties if we were to divide the length by the width.
![]()
![]()
![]()
![]()
To express this uncertainty in absolute form, we have to determine 2.7% of 1.78
![]()
So the result can also be written as
![]()
Significant Figures
An important factor in the accuracy and precision of measurements involves the precision of the measuring tool. In general, a precise measuring tool can measure values in very small increments. For example, a standard ruler can measure length to the nearest millimeter, while a caliper can measure length to the nearest 0.01 millimeter. The caliper is a more precise measuring tool because it can measure extremely small differences in length. The more precise the measuring tool, the more precise the measurements can be.
When we express measured values, we can only list as many digits as we initially measured with our measuring tool. For example, if you use a standard ruler to measure the length of a stick, you may measure it to be 36.7cm. You could not express this value as 36.71cm because your measuring tool was not precise enough to measure a hundredth of a centimeter. It should be noted that the last digit in a measured value has been estimated in some way by the person performing the measurement. For example, the person measuring the length of a stick with a ruler notices that the stick length seems to be somewhere in between 36cm and 37cm and he or she must estimate the value of the last digit. Using the method of significant figures, the rule is that the last digit written down in a measurement is the first digit with some uncertainty. To determine the number of significant digits in a value, start with the first measured value at the left and count the number of digits through the last digit written on the right. For example, the measured value 36.7cm has three significant digits or significant figures. Significant figures indicate the precision of a measuring tool that was used to measure a value.
Zeros
Special consideration is given to zeros when counting significant figures. The zeros in 0.053 are not significant, because they are only place-keepers that locate the decimal point. There are two significant figures in 0.053. The zeros in 10.053 are not place-keepers; they are significant. This number has five significant figures. The zeros in 1300 may or may not be significant. They could mean the number is known to the last digit, or they could be place-keepers. So 1300 could have two, three, or four significant figures. (To avoid this ambiguity, write 1300 in scientific notation.) Zeros are significant except when they serve only as place-keepers.
Example 1.4
Determine the number of significant figures in the following measurements:
- 0.0009
- 15,450.0
- 6×103
- 87.990
- 30.42
(a) 1; the zeros in this number are place-keepers that indicate the decimal point
(b) 6; here, the zeros indicate that a measurement was made to the 0.1 decimal point, so the zeros are significant
(c) 1; the value 103 signifies the decimal place, not the number of measured values
(d) 5; the final zero indicates that a measurement was made to the 0.001 decimal point, so it is significant
(e) 4; any zeros located in between significant figures in a number are also significant
Significant Figures in Calculations
When combining measurements with different degrees of accuracy and precision, the number of significant digits in the final answer can be no greater than the number of significant digits in the least precise measured value. There are two different rules, one for multiplication and division and the other for addition and subtraction, as discussed below.
1. For multiplication and division: The result should have the same number of significant figures as the quantity having the least significant figures entering into the calculation.
For example, the area of a circle can be calculated from its radius using A=πr2. Let us see how many significant figures the area has if the radius has only two—say, r=1.2m. Then,
![]()
even though π is good to at least eight digits.
2. For addition and subtraction: The answer can contain no more decimal places than the least precise measurement. Suppose that you buy 7.56-kg of potatoes in a grocery store as measured with a scale with a precision of 0.01 kg. Then you drop off 6.052-kg of potatoes at your laboratory as measured by a scale with a precision of 0.001 kg. Finally, you go home and add 13.7 kg of potatoes as measured by a bathroom scale with a precision of 0.1 kg. How many kilograms of potatoes do you now have, and how many significant figures are appropriate in the answer? The mass is found by simple addition and subtraction:
Next, we identify the least precise measurement: 13.7 kg. This measurement is expressed to the 0.1 decimal place, so our final answer must also be expressed to the 0.1 decimal place. Thus, the answer is rounded to the tenths place, giving us
![]()
Attributions
This chapter contains material taken from “Openstax College Physics-Introduction to Science and the Realm of Physics, Physical Quantities, and Units” by Openstax and is used under a CC BY 4.0 license. Download this book for free at Openstax-College Physics
Example 1.2 is adapted from Body Physics: Motion to Metabolism, by Lawrence Davis and used under a CC BY-NC-SA 4.0 International License.
To see what was changed, refer to the List of Changes.
