Electrostatics I – Electric Charge, Force, and Field
Last Update: 06/18/2022
Electric charge
You have probably experienced the phenomenon of static electricity: When you first take clothes out of a dryer, many (not all) of them tend to stick together; for some fabrics, they can be very difficult to separate. Another example occurs if you take a woolen sweater off quickly—you can feel (and hear) the static electricity pulling on your clothes, and perhaps even your hair. If you comb your hair on a dry day and then put the comb close to a thin stream of water coming out of a faucet, you will find that the water stream bends toward (is attracted to) the comb.
Many of the characteristics of static electricity can be explored by rubbing things together. Rubbing creates the spark you get from walking across a wool carpet, for example. Static cling generated in a clothes dryer and the attraction of straw to recently polished amber also result from rubbing. Similarly, lightning results from air movements under certain weather conditions. You can also rub a balloon on your hair, and the static electricity created can then make the balloon cling to a wall. We also have to be cautious of static electricity, especially in dry climates. When we pump gasoline, we are warned to discharge ourselves (after sliding across the seat) on a metal surface before grabbing the gas nozzle. Attendants in hospital operating rooms must wear booties with aluminum foil on the bottoms to avoid creating sparks that may ignite the oxygen being used.
Some of the most basic characteristics of static electricity include:
- The effects of static electricity are explained by a physical quantity not previously introduced, called electric charge.
- There are only two types of charge, one called positive and the other called negative.
- Like charges repel, whereas unlike charges attract.
- The force between charges decreases with distance.
How do we know there are two types of electric charge? When various materials are rubbed together in controlled ways, certain combinations of materials always produce one type of charge on one material and the opposite type on the other. By convention, we call one type of charge “positive”, and the other type “negative.” For example, when glass is rubbed with silk, the glass becomes positively charged and the silk negatively charged. Since the glass and silk have opposite charges, they attract one another like clothes that have rubbed together in a dryer. Two glass rods rubbed with silk in this manner will repel one another, since each rod has a positive charge on it. Similarly, two silk cloths so rubbed will repel, since both cloths have a negative charge.
Figure 20.1 shows a simple model of an atom with negative electrons orbiting its positive nucleus. The nucleus is positive due to the presence of positively charged protons. Nearly all charge in nature is due to electrons and protons, which are two of the three building blocks of most matter. (The third is the neutron, which is neutral, carrying no charge.) Other charge-carrying particles are observed in cosmic rays and nuclear decay and are created in particle accelerators. All but the electron and proton survive only a short time and are quite rare by comparison. An atom has an equal number of protons and electrons. Electrons carry the charge we have named negative. Protons carry an equal-magnitude charge that we call positive.

The charges of electrons and protons are identical in magnitude but opposite in sign. Therefore, an atom, as a whole, is electrically neutral. Usually, charges are formed by combinations of electrons and protons. The magnitude of this basic charge is
Electrons can be separated from the atom—for example, by rubbing materials together. Some atoms and molecules have a greater affinity for electrons than others and will become negatively charged by close contact in rubbing, leaving the other material positively charged.
No charge is actually created or destroyed when charges are separated as we have been discussing. Rather, existing charges are moved about. In fact, in all situations the total amount of charge is always constant. This universally obeyed law of nature is called the law of conservation of charge.
conductors and insulators
Some substances, such as metals and salty water, allow charges to move through them with relative ease. Some of the electrons in metals and similar conductors are not bound to individual atoms or sites in the material. These free electrons (conduction electrons) can move through the material much as air moves through loose sand. Any substance that has free electrons and allows charge to move relatively freely through it is called a conductor. The moving electrons may collide with fixed atoms and molecules, losing some energy, but they can move in a conductor. Superconductors allow the movement of charge without any loss of energy. Salty water and other similar conducting materials contain free ions that can move through them. An ion is an atom or molecule that has lost or gained additional electron(s), and as a result, it has a positive or negative (nonzero) total charge. In other words, in an ion, the total number of electrons is not equal to the total number of protons.
Other substances, such as glass, do not allow charges to move through them. These are called insulators. Electrons and ions in insulators are bound in the structure and cannot move easily. Pure water and dry table salt are insulators, whereas molten salt and salty water are conductors.
polarization of charge
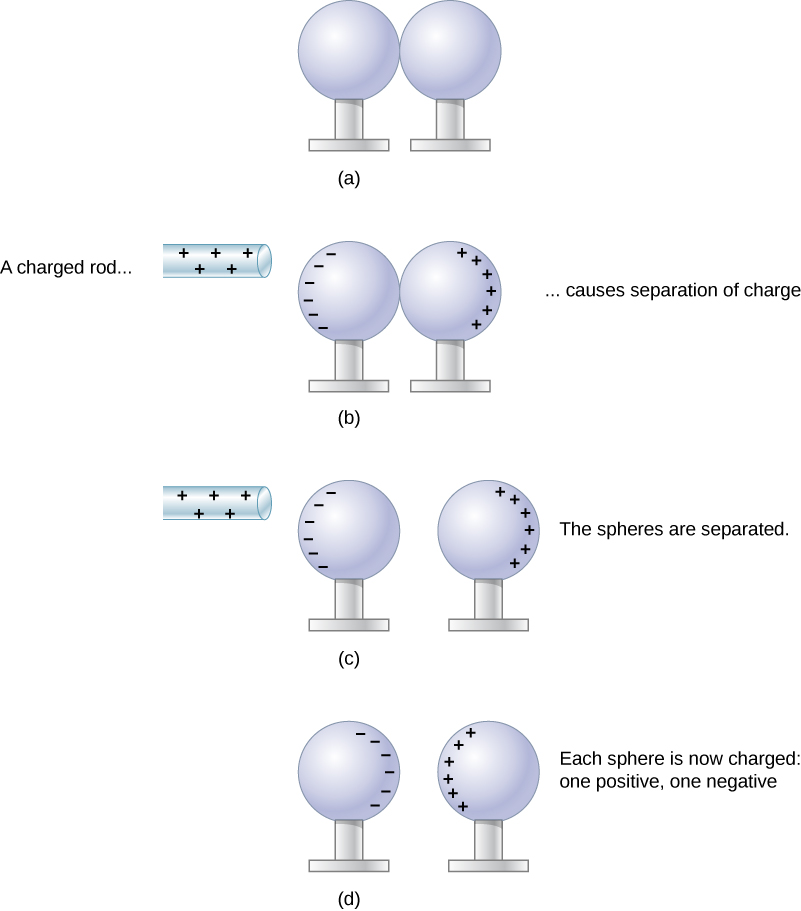
Let’s examine in more detail what happens in a conductor when an electrically charged object is brought close to it. As mentioned, the conduction electrons in the conductor are able to move with nearly complete freedom. As a result, when a charged insulator (such as a positively charged glass rod) is brought close to the conductor, the (total) charge on the insulator exerts an electric force on the conduction electrons. Since the rod is positively charged, the conduction electrons (which themselves are negatively charged) are attracted, flowing toward the insulator to the near side of the conductor. This is shown in Figure 20.2

Now, the conductor is still overall electrically neutral; the conduction electrons have changed position, but they are still in the conducting material. However, the conductor now has a charge distribution; the near end (the portion of the conductor closest to the insulator) now has more negative charge than positive charge, and the reverse is true of the end farthest from the insulator. The relocation of negative charges to the near side of the conductor results in an overall positive charge in the part of the conductor farthest from the insulator. We have thus created an electric charge distribution where one did not exist before. This process is referred to as inducing polarization—in this case, polarizing the conductor. The resulting separation of positive and negative charge is called polarization, and the material, or even the molecule, that exhibits polarization is said to be polarized.
Neutral objects can be attracted to any charged object. The pieces of straw attracted to polished amber are neutral, for example. If you run a plastic comb through your hair, the charged comb can pick up neutral pieces of paper. Figure 20.3 shows how the polarization of atoms and molecules in neutral objects results in their attraction to a charged object.
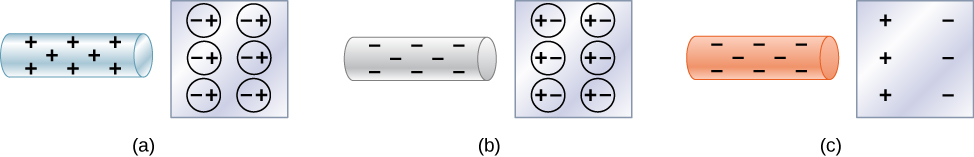
ways to transfer electrostatic charge to various materials (charging)
When a charged object comes in contact with a neutral object, some of the charges are transferred to the neutral object. This is called charging by contact.
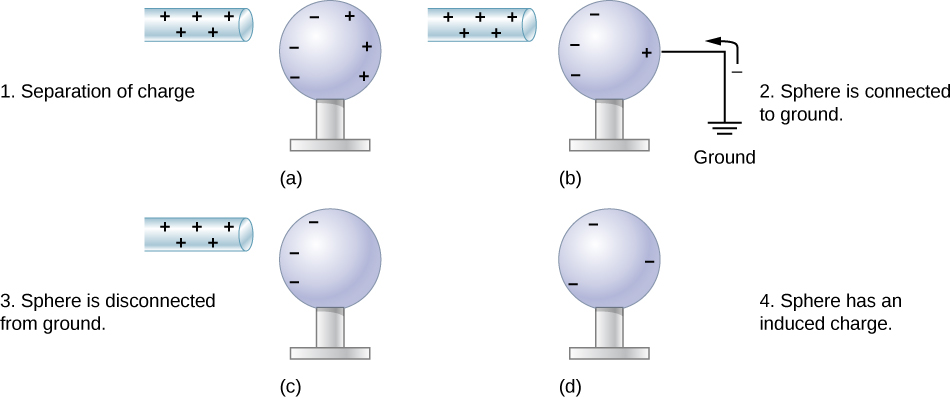
But there is another way to transfer charge from one object to a neutral conductor, without any direct contact between the charged object and the conductor. This is called charging by induction. The process of charging by induction is demonstrated in Figure 20.4.
Here are some cool things you can try yourself to see the interactions between charged objects.
coulomb’s law
Experiments with electric charges have shown that if two point objects each have an electric charge, then they exert an electric force on each other. The magnitude of the force is directly proportional to the net charge on each point object and inversely proportional to the square of the distance between them. The direction of the force vector is along the imaginary line joining the two point-objects and is dictated by the signs of the charges involved. These relationships are represented by the following equation known as Coulomb’s Law.
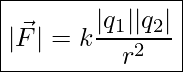 Coulomb’s Law
Coulomb’s Law
In this equation
![]() is the magnitude of the electric force between the two point-objects
is the magnitude of the electric force between the two point-objects
![]() is the magnitude of the charge of one point object
is the magnitude of the charge of one point object
![]() is the magnitude of the charge of the other point object
is the magnitude of the charge of the other point object
![]() is the distance between the two point-objects
is the distance between the two point-objects
![]() is a constant, called Coulomb’s constant,
is a constant, called Coulomb’s constant, ![]()
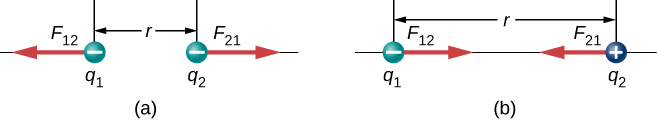
The direction of the force between the two point-charges depends on the type of charges that are interacting. Like charges repel, and unlike charges attract as shown in Figure 20.6
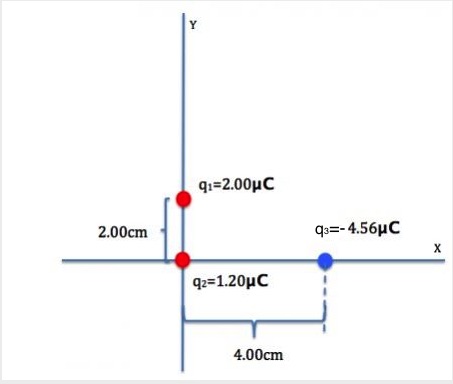
Example 20.1
Three point-charges are placed as shown in Figure 20.7. Determine the magnitude and direction of the net force on q1.
 |
 |
Solution
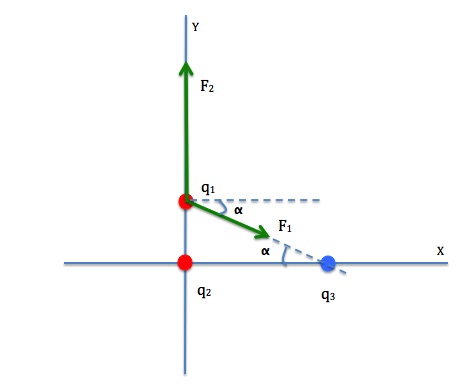
We need to use Coulomb’s law to find the force on q1 by q2, and the force on q1 by q3 and add them together using the method of vector addition.
Let’s label the force on q1 by q2 as ![]() and the distance between them as r2.
and the distance between them as r2.
![]()
![]()
![]()
Let’s label the force on q1 by q3 as ![]() and the distance between them as r1.
and the distance between them as r1.
We can use Pythagorean Theorem to find (r1 )2.
![]()
![]()
Now we can calculate ![]()
![]()
![]()
![]()
To add the two forces we must break them up into components and add the components. For a review of the analytical method of vector addition, see UNIT 4.
Let’s first calculate the angle ![]() .
. ![]() .
.
|
|
|
|
|
|
|
|
|
|
|
Let’s now find the magnitude and direction of the net force, ![]()

![]()
![]()
Finally, we determine the direction of the resultant force by finding Θ, the angle ![]() makes with the horizontal axis.
makes with the horizontal axis.
![]()
![]()
![]()
Therefore the net force on q1 is ![]()
Electric Forces In Biology
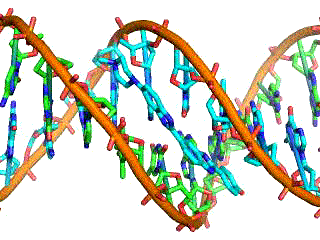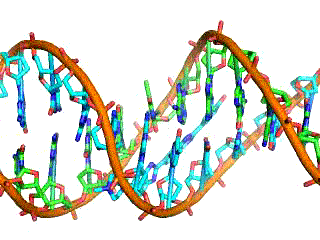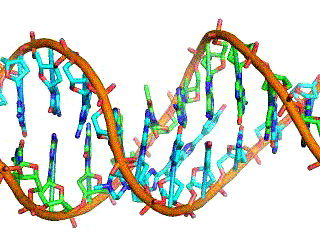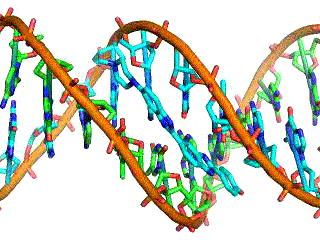
Classical electrostatics has an important role to play in modern molecular biology. Large molecules such as proteins, nucleic acids, and so on—so important to life—are usually electrically charged. DNA itself is highly charged; it is the electrostatic force that not only holds the molecule together but gives the molecule structure and strength. The figure below is a schematic of the DNA double helix.
The four nucleotide bases are given the symbols A (adenine), C (cytosine), G (guanine), and T (thymine). The order of the four bases varies in each strand, but the pairing between bases is always the same. C and G are always paired and A and T are always paired, which helps to preserve the order of bases in cell division (mitosis) so as to pass on the correct genetic information. Since the Coulomb force drops with distance, the distances between the base pairs must be small enough that the electrostatic force is sufficient to hold them together.
DNA is a highly charged molecule, with about 2e (fundamental charge) per 0.3nm. The distance separating the two strands that make up the DNA structure is about 1 nm, while the distance separating the individual atoms within each base is about 0.3 nm.
One might wonder why electrostatic forces do not play a larger role in biology than they do if we have so many charged molecules. The reason is that the electrostatic force is “diluted” due to screening between molecules. This is due to the presence of other charges in the cell.
The polarity of Water Molecules
The best example of this charge screening is the water molecule, represented as H2O. Water is a strongly polar molecule. Its 10 electrons (8 from the oxygen atom and 2 from the two hydrogen atoms) tend to remain closer to the oxygen nucleus than the hydrogen nuclei. This creates two centers of equal and opposite charges—what is called a dipole, as illustrated below. The magnitude of the dipole is called the dipole moment.

These two centers of charge will terminate some of the electric field lines coming from a free charge, as on a DNA molecule. This results in a reduction in the strength of the Coulomb interaction. One might say that screening makes the Coulomb force a short-range force rather than long-range.
Other ions of importance in biology that can reduce or screen Coulomb interactions are <span id="MathJax-Element-19-Frame" class="MathJax" style="overflow: initial;font-style: normal;font-weight: normal;line-height: normal;font-size: 14px;text-indent: 0px;text-align: left;text-transform: none;letter-spacing: normal;float: none;direction: ltr;max-width: none;max-height: none;min-width: 0px;min-height: 0px;border: 0px;padding: 0px;margin: 0px" role="presentation" data-mathml="“>Na+, and <span id="MathJax-Element-20-Frame" class="MathJax" style="overflow: initial;font-style: normal;font-weight: normal;line-height: normal;font-size: 14px;text-indent: 0px;text-align: left;text-transform: none;letter-spacing: normal;float: none;direction: ltr;max-width: none;max-height: none;min-width: 0px;min-height: 0px;border: 0px;padding: 0px;margin: 0px" role="presentation" data-mathml="“>K+, and <span id="MathJax-Element-21-Frame" class="MathJax" style="overflow: initial;font-style: normal;font-weight: normal;line-height: normal;font-size: 14px;text-indent: 0px;text-align: left;text-transform: none;letter-spacing: normal;float: none;direction: ltr;max-width: none;max-height: none;min-width: 0px;min-height: 0px;border: 0px;padding: 0px;margin: 0px" role="presentation" data-mathml="“>Cl–. These ions are located both inside and outside of living cells. The movement of these ions through cell membranes is crucial to the motion of nerve impulses through nerve axons.
Recent studies of electrostatics in biology seem to show that electric fields in cells can be extended over larger distances, in spite of screening, by “microtubules” within the cell. These microtubules are hollow tubes composed of proteins that guide the movement of chromosomes when cells divide, the motion of other organisms within the cell, and provide mechanisms for the motion of some cells (as motors).
Attribution
This chapter contains material taken from Openstax College Physics-Electric Charge and Electric Field, and Openstax University Physics Volume 2-Electric Charges and Fields, and is used under a CC BY 4.0 license. Download these books for free at Openstax
To see what was changed, refer to the List of Changes.
PROBLEMS
- [openstax univ. phys. vol.2 – 5.41 – modified] A 2.50-g copper penny is given a charge of -2.00×10-9C.
- How many excess electrons are on the penny?
- What is the mass of the excess electrons on the penny?
- [openstax univ. phys. vol. 2 – 5.45]A 50.0-g ball of copper has a net charge of 2.00μC. What fraction of the copper’s electrons has been removed? (Each copper atom has 29 protons, and copper has an atomic mass of 63.5.)
- [openstax univ. phys. vol. 2 – 5.49] Two charges+3.00μC and +12.0μC are fixed 1.00 m apart, with the second one to the right. Find the magnitude and direction of the net electrostatic force on a −2.00nC charge when placed at the following locations.
- Halfway between the two.
- Half a meter to the left of the +3.00μC charge.
- Half a meter above the +12.0μC charge in a direction perpendicular to the line joining the two fixed charges.
- [openstax univ. phys. vol. 2 – 5.50] In a salt crystal, the distance between adjacent sodium and chloride ions is 2.82×10-10m. What is the force of attraction between the two singly charged ions?
- [openstax univ. phys. vol.2 – 5.55] Two small balls, each of mass 5.00 g, are attached to silk threads 50.0 cm long, which are in turn tied to the same point on the ceiling, as shown below. When the balls are given the same charge Q, the threads hang at 5.00º to the vertical, as shown below. What is the magnitude of Q? What are the signs of the two charges?

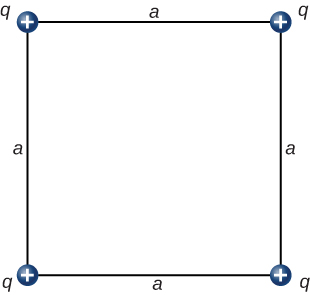
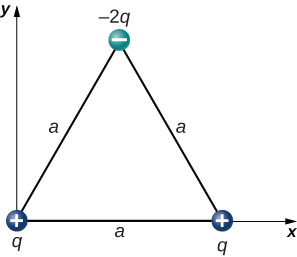
- [openstax univ. phys. vol. 2 – 5.60 – modified] In the configuration below, q=3.00nC, and a=4.00cm. What is the net electrostatic force on the charge located at the lower right-hand corner of the triangle shown here?
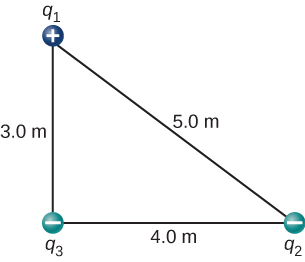
- [openstax univ. phys. vol. 2 – 5.62]The charges q1=2.00×10-7C, q2=-4.00×10-7C, and q3=-1.00×10-7C are placed at the corners of the triangle shown below. What is the net electrostatic force on q1?
- [openstax univ. phys. vol. 2 – 5.63 – modified] In the configuration below, q=8.00μC and a=5.00cm. What is the net electrostatic force on the charge q at the lower-right-hand corner of the square shown here?